SUMMARY
- Diagnosis of Growth Hormone Deficiency in Adults
- The insulin tolerance test (ITT) is the gold standard for diagnosing growth hormone (GH) deficiency (B).
- If ITT is contraindicated, perform two or more GH stimulation tests (GHRH-arginine, glucagon, levodopa, or clonidine) (B).
- Normal IGF-1 levels do not exclude GH deficiency. Low IGF-1 may suggest GH deficiency, unless the patient has poorly controlled diabetes, chronic liver disease, or uses oral contraceptives (C).
- GH deficiency can be diagnosed without stimulation tests in patients with typical clinical features, deficiencies in three or more pituitary hormones, and low IGF-1 (B).
- Repeat GH stimulation testing in adults with childhood-onset GH deficiency is needed unless there’s a proven genetic cause or irreversible pituitary damage (B).
- Repeated GH stimulation tests are not needed in adults with irreversible pituitary damage (B).
- Treatment of Growth Hormone Deficiency in Adults
- GH therapy is recommended for GH-deficient patients unless contraindicated. Start with a low dose, considering age, sex, and estrogen levels (A).
- Adjust GH dose based on clinical improvement, side effects, and target IGF-1 levels within the age-adjusted normal range (A).
- Monitor IGF-1 monthly or bimonthly during dose adjustment, then twice yearly at maintenance. Monitoring should include clinical response, side effects, and IGF-1 levels (B).
- Diagnosis and Treatment of Growth Hormone Deficiency in Children and Adolescents
- Perform two or more GH stimulation tests when suspecting GH deficiency in children (A).
- Repeated GH stimulation tests are not needed in children with pituitary lesions or proven genetic causes of GH deficiency (C).
- Continue GH replacement in children and adolescents until epiphyseal plates close or full height is reached (C).
- Resume GH replacement as soon as possible during transition in patients with GH deficiency (B).
- Benefits of Growth Hormone Treatment
- GH treatment improves body composition, exercise capacity, and bone mineral density in GH-deficient patients (A).
- GH treatment reduces cardiovascular disease risk in GH-deficient patients, but mortality reduction evidence is limited (B).
- GH treatment improves quality of life in GH-deficient patients (A).
- Risks and Side Effects of Growth Hormone Treatment
- GH treatment is contraindicated in active malignancy (except basal cell or squamous cell skin cancers) (A).
- Monitor blood glucose levels during GH treatment in diabetic patients; medication adjustments may be needed (B).
- Monitor thyroid and adrenal gland function during GH treatment in hypopituitarism patients (B).
INTRODUCTION
Growth hormone (GH) deficiency, a condition arising from inadequate production or action of growth hormone, can manifest in both childhood and adulthood. This deficiency can stem from congenital factors, present from birth, or acquired conditions developing later in life. Childhood-onset GH deficiency can be further classified into congenital, acquired, or idiopathic (unknown cause). Adult-onset GH deficiency is typically acquired, although it can also be a continuation of childhood-onset GH deficiency. Congenital causes include genetic mutations affecting GH synthesis, GH receptors, and structural brain abnormalities during development. Acquired GH deficiency is often linked to pituitary and hypothalamic tumors, treatments for these tumors like surgery or radiation, infiltrative diseases, vascular damage, and infections (Table 1). These factors disrupt the delicate hormonal balance, leading to a cascade of physiological changes.
Table 1. Causes of Growth Hormone Deficiency
| Congenital | Acquired |
|---|---|
| Genetic Factors | Neoplastic Conditions |
| Transcription factor defects (PIT-1, PROP-1, LHX3/4, HESX-1, PITX-2) | Pituitary adenoma |
| GHRH receptor gene defects | Craniopharyngioma |
| GH secretagogue receptor gene defects | Rathke’s cleft cyst |
| GH gene defects | Glioma/astrocytoma |
| GH receptor/post receptor defects | Germinoma |
| Metastatic Tumors | |
| Brain Structural Defects | Infiltrative/Granulomatous Diseases |
| Agenesis of corpus callosum | Langerhans cell histiocytosis |
| Septo-optic dysplasia | Sarcoidosis |
| Empty sella syndrome | Hypophysitis |
| Holoprosencephaly | |
| Encephalocele | Vascular Damage |
| Hydrocephalus | Head injury |
| Arachnoid cyst | Pituitary tumor apoplexy |
| Sheehan’s syndrome | |
| Midline Facial Defects | Subarachnoid hemorrhage |
| Single central incisor | |
| Cleft lip/palate | Treatment of Pituitary and Hypothalamic Diseases |
| Cranial irradiation | |
| Surgery of the pituitary or hypothalamus | |
| Central Nervous System Infection | |
| Idiopathic Causes |
Modified from Molitch et al. [^1]; and Melmed [^2], with permission from Massachusetts Medical Society.
PIT-1, pituitary transcription factor-1; PROP-1, prophet of pit-1; LHX3/4, LIM class homeobox transcription factor Lhx3, 4; HESX-1, homeobox-1; PITX-2, paired-like homeodomain transcription factor-2; GHRH, growth hormone-releasing hormone; GH, growth hormone.
Hypothalamic-pituitary tumors, or their treatments, are the most frequent cause of adult-onset GH deficiency. Pituitary macroadenomas are associated with at least one pituitary hormone deficiency in 30% to 60% of cases [^3]. Hormone deficiencies often arise from disrupted blood flow due to pressure on the pituitary stalk’s portal veins [^4]. While surgery to remove pituitary tumors can lead to a 50% recovery of tumor-induced hypopituitarism [^5], GH is the least likely pituitary hormone to recover [^4]. Radiation therapy is also a significant cause of GH deficiency, with younger patients and longer post-radiation time being at higher risk [^6]. Radiation doses exceeding 40 Gy carry a 50% risk of GH deficiency [^7].
GH deficiency is also observed in 25% of patients after head injury or subarachnoid hemorrhage [^8, ^9]. Pituitary hormone levels in these patients require monitoring upon admission and regularly thereafter, as some may recover while others deteriorate.
Clinically, GH deficiency manifests with symptoms such as increased body fat, reduced muscle mass, fatigue, and diminished quality of life [^10]. However, symptoms like fatigue and weakness are nonspecific, making accurate diagnosis challenging. To address these challenges and ensure appropriate diagnosis and treatment, the Korean Endocrine Society developed guidelines for Gh Deficiency Diagnosis and management, particularly relevant in the context of increasing cases and insurance reimbursement considerations in South Korea. This article aims to provide an expert guide on GH deficiency diagnosis based on these guidelines, focusing on clinically relevant aspects for healthcare professionals in English-speaking regions.
METHODS FOR GUIDELINE DEVELOPMENT
The Korean Endocrine Society’s Insurance Committee, in collaboration with the Korean Neuroendocrine Study Group and the Korean Society of Pediatric Endocrinology, developed these guidelines. Given the absence of pre-existing diagnostic guidelines for GH deficiency in Korea and limited domestic research, the recommendations were formulated through expert panel discussions and a comprehensive review of existing international literature. Where evidence was lacking, recommendations were based on expert consensus. Disagreements were resolved through majority voting. Each recommendation’s strength was graded based on the quality of supporting evidence, as outlined in Table 2.
Table 2. Definition of Recommendation Levels
| Recommendation Level | Definition |
|---|---|
| A | Strong Recommendation: Clear rationale supported by multiple randomized controlled trials with generalizable and robust meta-analysis results. |
| B | Moderate Recommendation: Reliable basis supported by well-conducted cohort or patient-control group studies. |
| C | Weak Recommendation: Possible basis from randomized clinical studies, case reports, or small-scale observational studies with inherent limitations. |
| E | Expert Opinion: Recommendations based on expert opinion or clinical experience, lacking direct supporting evidence. |
DISCUSSION OF DIAGNOSTIC RECOMMENDATIONS
1. Diagnosis of Growth Hormone Deficiency in Adults
GH deficiency should be considered in adults presenting with suggestive clinical features such as increased body fat, decreased muscle mass, lethargy, and reduced quality of life. Diagnosis relies on GH stimulation tests. Evaluation is particularly important in patients with conditions known to cause GH deficiency, as nonspecific symptoms can lead to misdiagnosis. It is essential to address other hormone deficiencies before assessing GH secretion. Given GH’s pulsatile release, GH stimulation tests are crucial. These tests involve administering agents like insulin (in ITT), growth hormone-releasing hormone (GHRH), arginine, glucagon, levodopa, or clonidine, followed by serial blood GH level measurements. The peak GH level achieved is used for interpretation. While no single test is universally considered the gold standard, the ITT is a major GH stimulation test. GHRH is no longer available in South Korea and the United States. Table 3 summarizes GH stimulation tests, including procedures, GH cut-points, and considerations.
Table 3. Dynamic Tests for Diagnosing GH Deficiency
| Hormone Test | Procedure | GH Cut-points (µg/L) | Considerations |
|---|---|---|---|
| Insulin Tolerance Test (ITT) | Insulin 0.05–0.15 U/kg IV. Sample blood at -30, 0, 30, 60, 120 min for GH and glucose. | >5.0 (AACE, 2019) >3–5 (ES, 2016) | Glucose should drop. Contraindications: seizures, coronary artery disease, pregnancy, age >65 yr. |
| GHRH-Arginine Test | GHRH 1 µg/kg (max 100 µg) IV, then arginine infusion 0.5 g/kg (max 35 g) over 30 min. Sample blood at 0, 30, 45, 60, 75, 90, 105, 120 min for GH. | >4 (Cutoffs should correlate to BMI) | False normal GH response possible in hypothalamic damage. |
| Glucagon Stimulation Test | Glucagon 1 mg (1.5 mg if weight >90 kg) IM. Sample blood at 0, 30, 60, 90, 120, 150, 180, 210, 240 min for GH and glucose. | >3 (if BMI ≥25 kg/m²) >1 (if BMI ≥25 kg/m²) | Obesity may blunt GH response. Contraindications: severe fasting hyperglycemia (>180 mg/dL). Side effects: nausea, vomiting, headache, delayed hypoglycemia. |
| Levodopa Stimulation Test | Levodopa 500 mg PO. Sample blood at 0, 60, 90, 120 min for GH. | >3 | Side effects: nausea, vomiting, dizziness, headache. |
| Clonidine Stimulation Test | Clonidine 0.15 mg/m² (max 0.25 mg) PO. Sample blood at 0, 30, 60, 90, 120 min for GH. | >3 | Side effects: hypotension, drowsiness. Contraindications: coronary artery disease history. |
| Macimorelin Test | Macimorelin 0.5 mg/kg oral solution. | >2.8 | Avoid use with QT-prolonging drugs. May not accurately diagnose hypothalamic disease. Side effect: mild dysgeusia. |
GH, growth hormone; ITT, insulin-tolerance test; IV, intravenous; AACE, American Association of Clinical Endocrinologists; ES, Endocrine Society; GHRH, growth hormone-releasing hormone; BMI, body mass index; GHD, growth hormone deficiency; IM, intramuscular; PO, per os.
1.1. Insulin Tolerance Test (ITT) as the Primary Diagnostic Test
The ITT is recommended as the standard diagnostic test for GH deficiency. It is well-validated, but its induction of hypoglycemia poses risks, making it contraindicated in elderly patients and those with epilepsy or cardiovascular disease [^11]. Even in healthy individuals, continuous monitoring is necessary during ITT. Obese patients with insulin resistance require higher insulin doses to achieve hypoglycemia, increasing the risk of delayed hypoglycemia. Furthermore, ITT results can vary even in the same individual and are influenced by factors like the menstrual cycle, making reproducibility challenging.
1.2. Alternative GH Stimulation Tests
When ITT is contraindicated, two or more alternative GH stimulation tests, such as the GHRH-arginine, glucagon, levodopa, or clonidine tests, should be used. Aimaretti et al. [^12] reported that combining arginine, which suppresses hypothalamic somatostatin, with GHRH effectively and safely stimulates GH. Biller et al. [^13] compared five GH stimulation tests and found that at a GH level of 5.1 μg/L, ITT had 96% sensitivity and 92% specificity. The GHRH-arginine test showed similar diagnostic validity at a GH level of 4.1 μg/L, with 95% sensitivity and 91% specificity. However, GHRH directly stimulates the pituitary and may yield false negatives in hypothalamic disorders or post-radiation patients. GH cut-offs for the GHRH-arginine test need adjustment for age and BMI [^14, ^15]. While higher BMI can slightly lower GH response in ITT, adjusting GH cut-offs is not recommended [^16]. Arginine-only tests, with a GH cut-off of 0.4 μg/L, lack diagnostic accuracy and are not advised.
The glucagon stimulation test indirectly stimulates GH via insulin secretion, resulting in a delayed GH response requiring a minimum 3-hour test duration and a risk of delayed hypoglycemia. Intramuscular or subcutaneous glucagon injection is more effective than intravenous. A GH cut-off of 3 μg/L is generally adequate, but in obese patients (BMI >25 kg/m²) with reduced GH sensitivity, a 1 μg/L cut-off is suggested [^17–^21]. Although higher blood glucose levels may weaken GH response [^22], specific diagnostic GH cut-offs based on blood glucose levels are not yet defined. Side effects include nausea, vomiting, and headaches; severe hypotension, hypoglycemia, and convulsions have been reported in elderly patients.
Levodopa and clonidine are weaker GH stimulators, acting through dopamine and alpha receptors in the hypothalamus, respectively. They require sensitive GH measurement methods for accurate diagnosis. Optimal GH cut-off values based on age, sex, BMI, blood glucose, or underlying disorders are not well-established.
Macimorelin, a recently approved oral GH secretagogue receptor-1a agonist, shows similar sensitivity (92%) and specificity (96%) to ITT with fewer side effects [^23]. A common side effect is mild taste disturbance, while a severe side effect is QT interval prolongation. Macimorelin is not yet available in South Korea.
Analytical methods for GH measurement significantly affect diagnostic GH cut-offs. Accurate GH measurement is crucial. Blood GH comprises various isoforms and isomers, primarily 22 and 20 kDa forms. GH-binding proteins can bind up to 50% of blood GH, potentially interfering with immunoassays. Variations in GH measurement methods across institutions highlight the importance of using the GH calibration standard 98/574 from the National Institute for Biological Standards and Control and highly purified recombinant pituitary GH [^24]. Manufacturers should specify assay validity, GH isoforms measured, analytes, antibodies used, and interference from GH-binding proteins.
1.3. Role of IGF-1 Levels in GH Deficiency Diagnosis
Normal IGF-1 levels do not rule out GH deficiency [^13, ^25–^27]. Higher BMI is associated with weaker GH response and increased IGF-1 levels [^28]. However, low IGF-1 in obese patients can indicate GH deficiency.
1.4. GH Deficiency Diagnosis Without Stimulation Tests
GH stimulation tests can be omitted if patients exhibit very low levels of three or more pituitary hormones along with low IGF-1 (at least 2.0 standard deviations below normal), structural hypothalamic-pituitary conditions, genetic conditions affecting the hypothalamic-pituitary axis, or structural lesions in the hypothalamus or pituitary gland [^1, ^25, ^29].
1.5. Repeat GH Stimulation Testing in Childhood-Onset GH Deficiency
Repeat GH stimulation testing is recommended in adults with childhood-onset GH deficiency, unless a genetic cause or irreversible pituitary damage is confirmed. Many patients with idiopathic childhood-onset GH deficiency show normal GH secretion when re-evaluated in adulthood [^30].
1.6. No Repeat Testing in Irreversible Pituitary Damage
GH deficiency due to structural conditions like tumors, surgery, radiation, or genetic disorders is typically irreversible. Therefore, repeated GH stimulation tests are not routinely needed in adults with irreversible pituitary damage [^31].
2. Treatment of Growth Hormone Deficiency in Adults
2.1. Initiation of GH Therapy
GH replacement therapy is recommended for GH-deficient adults diagnosed via stimulation tests, unless contraindicated. Therapy should commence at low doses due to dose-dependent side effects [^32]. Common side effects include fluid retention, arthralgia, muscle pain, sensory disturbances, carpal tunnel syndrome, sleep apnea, sleep disorders, and dyspnea, affecting approximately 20% of patients, and are more frequent in elderly, obese, and female patients, but often resolve with dose reduction [^33]. Individualized dosing, rather than weight-based, reduces side effects by half [^34].
2.2. GH Dose Adjustment and Monitoring
Appropriate GH therapeutic levels are age-dependent, lower in the elderly and higher in younger individuals [^35]. For ages 30-60, a starting dose of 0.2-0.3 mg/day (0.8-1.2 IU/day) is suitable. Younger patients (<30 years) may start at 0.4-0.5 mg/day (1.6-2.0 IU/day), while older patients (>60 years) should start at 0.1-0.2 mg/day (0.4-0.8 IU/day), with gradual increases (Fig. 1). Dosage should be increased monthly or bimonthly by 0.1-0.2 mg/day (0.4-0.8 IU/day), guided by clinical response, side effects, and age-adjusted IGF-1 levels [^1]. While targeting normal IGF-1 levels is common, the evidence supporting specific targets is still evolving. Clinical responses are typically seen after 6 months of treatment.
Fig. 1. Algorithm for Growth Hormone Therapy in GH Deficient Adults
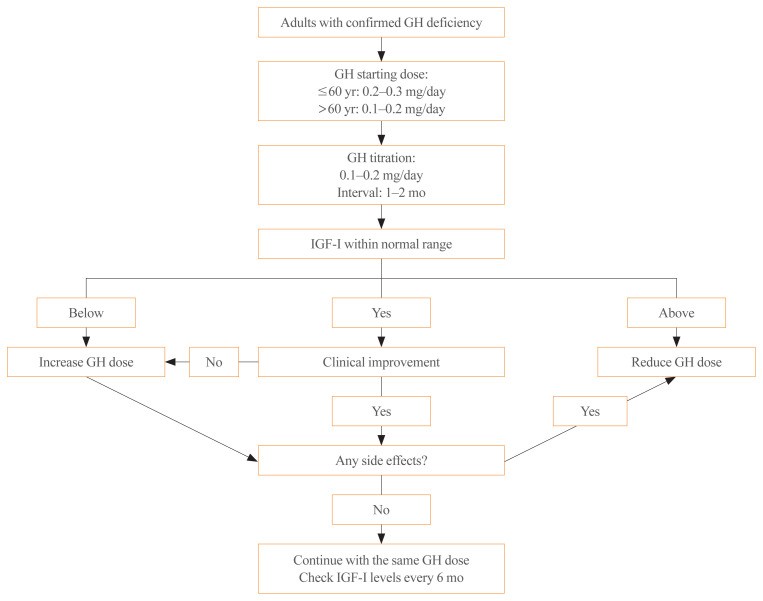 Algorithm for Growth Hormone Therapy in GH Deficient Adults
Algorithm for Growth Hormone Therapy in GH Deficient Adults
IGF-1, insulin-like growth factor-1.
Females often exhibit higher GH resistance, requiring higher starting and maintenance doses [^36]. Estrogen stimulates suppressor of cytokine signaling 2 (SOCS2), a GH suppressor in the liver [^37], where 85% of serum IGF-1 originates. Oral estrogen suppresses IGF-1, necessitating higher GH doses in females [^37]. Even at similar IGF-1 levels, GH effects on body fat, LDL cholesterol, and bone turnover markers are less pronounced in females [^38]. Switching from oral to transdermal estrogen reduces GH requirements [^39].
2.3. Monitoring During GH Therapy
During dose adjustment, monitor IGF-1 levels monthly or bimonthly, along with clinical response and side effects [^1]. Once maintenance dose is established, monitor IGF-1 every six months (Fig. 1). Annual lipid and fasting glucose tests are recommended. Bone mineral density should be re-evaluated every 1.5-2 years if initially abnormal. Waist circumference and quality of life should also be assessed. Adjustments to thyroid or adrenal hormone doses may be needed after GH initiation. If no clear response to GH replacement is observed after at least one year, treatment suspension can be considered [^1].
Long-acting GH formulations, administered weekly or monthly, are available. Meta-analysis suggests similar efficacy and safety to daily GH [^40]. However, IGF-1 levels were significantly elevated in children on long-acting GH. Further research is needed to optimize long-acting GH regarding peak and trough levels, dose adjustment, IGF-1 monitoring frequency, and long-term cost-effectiveness compared to daily GH [^41].
3. Diagnosis and Treatment of Growth Hormone Deficiency in Children and Adolescents
3.1. Diagnostic GH Stimulation Tests in Children
Childhood-onset GH deficiency can persist into adulthood. Diagnostic workup includes auxology, bone age assessment, IGF-1 and IGF binding protein 3 measurements, GH stimulation tests, brain imaging, and genetic tests if necessary [^42]. GH stimulation tests are vital but invasive and carry side effects [^43]. Their validity and reproducibility have been questioned [^44, ^45]. Therefore, diagnosing GH deficiency in children typically requires two or more GH stimulation tests. GH replacement for children is usually 6-7 times weekly at 22-35 μg/kg/day (0.16-0.24 mg/kg/week) or 12 IU (4 mg) per body surface area (m²/week) via subcutaneous injection until epiphyseal plate closure [^46].
3.2. When Repeat GH Stimulation Tests Are Not Required
Re-evaluation of GH status with repeat stimulation tests during adolescence is debated. For patients with high likelihood of persistent GH deficiency, re-evaluation is unnecessary [^25]. Patients with structural lesions in the hypothalamus or pituitary, such as tumors, have high risk of persistent GH deficiency [^47, ^48]. Certain genetic defects also lead to irreversible GH deficiency [^49]. Thus, repeat GH stimulation tests are not needed in patients with confirmed genetic or structural pituitary abnormalities.
3.3. Duration of GH Replacement in Children and Adolescents
GH replacement should continue in children and adolescents until epiphyseal plates close or final height is achieved. Benefits extend beyond height gain. Childhood-onset GH deficiency contributes to low bone mass and increased fracture risk in adulthood [^50], and bone mass deficits persist at diagnosis and final height [^51]. Resuming GH replacement improves body composition, with increased muscle mass and decreased body fat [^35]. Dyslipidemia deterioration has been reported after GH cessation in childhood-onset GH deficiency [^52], and cardiac dimension reduction has also been observed [^53]. Therefore, maintaining GH replacement until growth completion is crucial for overall health.
3.4. Importance of Timely GH Replacement Resumption During Adolescence
Appropriate GH replacement during adolescence is essential [^54] to maintain hormonal care continuity and prevent health problems. Dropout risk is higher in GH-deficient patients who discontinue GH during transition [^54]. One study showed 21% of pubertal patients discontinued GH without full evaluation, and 18% were lost to follow-up [^55]. Standardized transition strategies and referral systems are needed [^56]. If GH replacement is stopped, it should be restarted as soon as possible.
4. Benefits of Growth Hormone Treatment
4.1. Improvements in Body Composition, Exercise Capacity, and Bone Mineral Density
GH deficiency can lead to decreased bone mineral density, muscle strength, exercise capacity, impaired memory, reduced physical activity and vitality, fatigue, concentration difficulties, and sleep disorders [^57, ^58]. Patients are also at higher cardiovascular risk due to central obesity, dyslipidemia, and insulin resistance [^59–^61].
GH treatment consistently improves body composition, reducing fat mass and increasing muscle mass [^62, ^63], preferentially visceral fat [^64], and lean body mass [^27, ^65]. Improvements in muscle function [^65] and lipid metabolism [^66] are also expected. Korean studies confirm GH treatment reduces weight, body fat, and insulin resistance [^67–^70].
Adults with GH deficiency have lower bone mineral density than age- and sex-matched controls [^71–^73], and bone loss severity correlates with GH deficiency severity [^74]. Fracture risk is 2- to 5-fold higher in GH-deficient patients [^75, ^76]. GH treatment significantly improves bone mineral density, particularly in males and those with severe bone loss [^77], and prevents fractures even in patients without prior osteoporosis [^78].
4.2. Cardiovascular Disease Risk Reduction
GH deficiency increases cardiovascular disease risk through adverse effects on metabolic parameters like abdominal obesity, insulin resistance, dyslipidemia, and increased inflammatory markers [^79]. It also negatively impacts heart function and atherosclerosis, causing reduced left ventricular thickness, impaired ejection fraction, and diastolic filling [^80]. Cardiovascular mortality is twice as high in GH-deficient adults [^81]. Svensson et al. [^82] reported lower fatal myocardial infarction and mortality rates with GH replacement in hypopituitarism patients [^83]. A meta-analysis also showed reduced mortality in GH-treated hypopituitary men [^84], and Korean studies show reduced inflammation markers with GH replacement [^70]. GH replacement is expected to benefit cardiovascular health. However, mortality improvement is less pronounced in women [^80], and whether GH replacement fully reverses long-term cardiovascular risk factors is unclear [^83]. More long-term, large-scale research is needed [^85].
4.3. Enhanced Quality of Life
GH deficiency impairs quality of life due to metabolic and physical deterioration and increased cardiovascular risk. Studies show poorer sleep, social integration, and physical activity, with physical health negatively impacting occupation and daily life [^86]. Svensson et al. [^82] found GH replacement improves quality of life, supported by Korean studies showing improved quality of life in older women with GH replacement due to increased bone mineral density, lean body mass, muscle strength, and reduced body fat [^87, ^88].
5. Risks and Side Effects of Growth Hormone Treatment
Common GH treatment side effects include peripheral edema, joint pain, carpal tunnel syndrome, sensory disturbances, and increased blood glucose [^33, ^89], more prevalent in elderly or obese patients and those overtreated with GH [^33]. Side effects can persist for 3+ years [^90]. The potential for GH to worsen malignancies requires careful consideration.
5.1. Contraindication in Active Malignancy
Theoretically, increased IGF-1 axis activity from GH treatment could exacerbate malignancies, as GH and IGF-1 are linked to various cancers [^91]. GH treatment is contraindicated in active malignancies, except basal and squamous cell skin cancers. This is based on theoretical risk of tumor growth, with limited clinical evidence [^92]. Research on GH treatment and cancer recurrence or secondary tumors in GH-deficient patients focuses on childhood cancer survivors [^93–^95]. Some studies reported slightly increased secondary tumor risk in childhood cancer survivors treated with GH [^94, ^95], but most show no link between GH treatment and cancer recurrence or secondary tumors [^96–^103]. Stable residual benign intracranial tumors do not contraindicate GH treatment. GH treatment can be considered in patients with a malignancy history in remission for at least one year without recurrence [^104].
5.2. Blood Glucose Monitoring in Diabetes Mellitus
GH and IGF-1 influence insulin resistance and pancreatic β-cell insulin secretion [^105]. Both high and low GH/IGF-1 can cause blood glucose abnormalities. Acromegaly and GH deficiency are associated with elevated and reduced blood glucose, respectively. Large-scale studies link GH treatment to insulin resistance and type 2 diabetes [^106]. Insulin sensitivity changes with GH treatment vary based on body composition, age, and genetics. A randomized controlled trial by Hoffman et al. [^27] showed a 13% prediabetes and 4% diabetes increase in the GH-treated group compared to placebo. Careful diabetes monitoring and anti-diabetic medication adjustments are needed during GH treatment.
5.3. Monitoring Thyroid and Adrenal Function in Hypopituitarism
GH deficiency often co-occurs with other hypopituitarism manifestations. Thyroid and adrenal function should be monitored during GH treatment, even if initially normal [^58]. Hypothyroidism reduces IGF-1 and GH secretion [^107, ^108]. Central hypothyroidism should be treated before assessing GH function. GH treatment can lower free thyroxine levels. 36-47% of GH-deficient patients with normal thyroid function develop hypothyroidism, and 16-18% of hypothyroid patients need increased levothyroxine doses within 3-6 months of GH treatment [^109, ^110]. Check for central hypothyroidism 6 weeks after GH initiation or dose change [^111].
Hypothalamic-pituitary-adrenal function should be assessed before and after GH treatment. GH suppresses 11β-hydroxysteroid dehydrogenase type 1, increasing the cortisone-to-cortisol ratio in GH deficiency [^108, ^112]. Adrenal insufficiency may be masked by GH deficiency. GH treatment in such patients requires careful monitoring for adrenal function deterioration [^113, ^114].
CONCLUSIONS
Despite ongoing debate about GH treatment’s impact on cardiovascular mortality in hypopituitarism, GH treatment for GH deficiency offers more benefits than risks. Accurate GH deficiency diagnosis via stimulation tests is crucial before initiating GH therapy. Individualized GH dosing is essential to minimize side effects and maximize efficacy. Regular monitoring of side effects and clinical response is necessary during maintenance therapy.
ACKNOWLEDGMENTS
This work was supported by the Korean Endocrine Society.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception or design: J.H.K., H.W.C., S.O.C., C.R.K., C.H.K., E.J.L. Acquisition, analysis, or interpretation of data: J.H.K., H.W.C., S.O.C., C.R.K., C.H.K., E.J.L. Drafting the work or revising: J.H.K., H.W.C., S.O.C., C.R.K., K.H.P., D.J.L., K.J.K., J.S.L., G.K., Y.M.C., S.H.A., M.J.J., Y.H., J.H.L., B.K.K., Y.J.C., K.A.L., S.S.M., H.Y.A., H.S.C., S.M.H., D.Y.S., J.A.S., S.H.K., S.O., S.H.Y., B.J.K., C.H.S., S.W.K., C.H.K., E.J.L. Final approval of the manuscript: J.H.K., H.W.C., S.O.C., C.R.K., C.H.K., E.J.L.
REFERENCES
[^1]: Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML, Endocrine Society. Evaluation and treatment of adult growth hormone deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Nov;96(11):3170-88. doi: 10.1210/jc.2011-1779. Epub 2011 Oct 3. PMID: 21965539.
[^2]: Melmed S. Acromegaly pathogenesis and diagnosis. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, editors. Williams textbook of endocrinology. 13th ed. Philadelphia: Elsevier Saunders; 2016. p. 273–303.
[^3]: Gillam MP, Molitch ME, Lombardi D, Odell WD, Hendelman J, Osamura RY, et al. Proliferation and spontaneous hormone secretion by human nonfunctioning pituitary adenomas: in vitro studies. J Clin Endocrinol Metab. 1993 Jul;77(1):167-75. doi: 10.1210/jcem.77.1.8392402. PMID: 8392402.
[^4]: Webb SM, Estrada J, Puig-Domingo M, Lugo G, Pena L, Romero V, et al. Hypopituitarism after clinically non-functioning pituitary adenoma surgery. Clin Endocrinol (Oxf). 2005 Jun;62(6):672-80. doi: 10.1111/j.1365-2265.2005.02275.x. PMID: 15941338.
[^5]: Roelfsema F, Pereira AM. The diagnosis and treatment of hypopituitarism in adults. J Clin Endocrinol Metab. 2015 Apr;100(4):1667-81. doi: 10.1210/jc.2014-4324. PMID: 25831443.
[^6]: Littley MD, Shalet SM, Beardwell CG, Ahmed SR, Sutton ML, Pearson DW. Hypopituitarism following external radiotherapy for pituitary tumours in adults. Q J Med. 1989 Nov;73(271):145-60. PMID: 2515237.
[^7]: Darzy KH, Shalet SM. Hypopituitarism after radiotherapy to the hypothalamus and pituitary. Pituitary. 2002;5(1):39-47. doi: 10.1023/a:1020846114357. PMID: 12439612.
[^8]: Daniel M, Verstichel P, Cottier JP, Philippin L, Lienhardt MM, Renard E, et al. Hypopituitarism after traumatic brain injury: prevalence and predictive factors. Am J Med. 2006 Dec;119(12):1012-9. doi: 10.1016/j.amjmed.2006.04.024. PMID: 17145283.
[^9]: Aimaretti G, Ambrosio MR,терые B, Benvenga S, Bizzarri C, Cannavò S, et al. Hypopituitarism after traumatic brain injury: a prospective study. J Clin Endocrinol Metab. 2005 Dec;90(12):6630-5. doi: 10.1210/jc.2005-1443. Epub 2005 Sep 27. PMID: 16192339.
[^10]: Rosen T, Bengtsson BA. Growth hormone and quality of life. Horm Res. 1991;36 Suppl 1:49-52. doi: 10.1159/000182361. PMID: 1765799.
[^11]: Swerdlow AJ, Higgins CD, Adlard P, Woodhouse LJ, Jones ME, Gillett GT, et al. Risks of diabetes mellitus, stroke, myocardial infarction, and cancer in patients with hypopituitarism. BMJ. 2007 Mar 10;334(7592):487. doi: 10.1136/bmj.39099.530677.55. Epub 2007 Feb 16. PMID: 17301123; PMCID: PMC1828157.
[^12]: Aimaretti G, Corneli G, Razzore P, Baffoni C, Arvat E, Bellone S, et al. Comparison between insulin and arginine plus growth hormone-releasing hormone tests for the diagnosis of growth hormone deficiency in adults. J Clin Endocrinol Metab. 1998 Jul;83(7):2566-70. doi: 10.1210/jcem.83.7.4955. PMID: 9661638.
[^13]: Biller BM, Luciano R, Colao A, Johannsson G, Lombardi G, Mallea-Gil S, et al. Diagnostic accuracy of insulin tolerance test, growth hormone-releasing hormone-arginine, and glucagon stimulation tests in the diagnosis of adult growth hormone deficiency. J Clin Endocrinol Metab. 2002 Jul;87(7):2064-70. doi: 10.1210/jcem.87.5.8481. PMID: 12107182.
[^14]: Janssen YJ, van de Lely AJ, van M.M. Growth hormone deficiency: diagnosis and treatment. Pituitary. 2008;11(3):241-52. doi: 10.1007/s11102-008-0117-x. PMID: 18587561; PMCID: PMC2518188.
[^15]: Gasco V, Corneli G, Rovere S, Maccario M, Bona G, Ghigo E, et al. Influence of age and body mass index on the growth hormone (GH) response to GH-releasing hormone plus arginine test in healthy subjects. J Clin Endocrinol Metab. 2001 Feb;86(2):607-11. doi: 10.1210/jcem.86.2.7159. PMID: 11158048.
[^16]: Gasco V, Gatta B, Martina V, Maccario M, Corneli G, Ghigo E, et al. Influence of body mass index on insulin-induced hypoglycaemia and growth hormone (GH) response in normal adults. Clin Endocrinol (Oxf). 2003 Feb;58(2):227-31. doi: 10.1046/j.1365-2265.2003.01720.x. PMID: 12580948.
[^17]: Popovic V, Micic D, Petrovic D, Djurovic M, Milic N, Dieguez C, et al. Glucagon stimulation test in assessment of growth hormone deficiency in adults: comparison with insulin tolerance test. Eur J Endocrinol. 2003 Jul;149(1):39-44. doi: 10.1530/eje.0.1490039. PMID: 12825970.
[^18]: Sherlock M, Reaven GM, Falahati E, Steffes M, King J, Toogood AA, et al. Glucagon stimulation test: effect of obesity and comparison with the insulin tolerance test. Eur J Endocrinol. 2009 Nov;161(5):783-9. doi: 10.1530/EJE-09-0544. Epub 2009 Aug 25. PMID: 19703986.
[^19]: Hartman ML, Guttmann IS, Vance ML, Thornton JE, Clasey JL, Kopelman HA, et al. Contribution of age and body composition to gender differences in growth hormone secretion in adults. J Clin Endocrinol Metab. 1996 Jul;81(7):2429-35. doi: 10.1210/jcem.81.7.8675551. PMID: 8675551.
[^20]: Ghigo E, Arvat E, Mazza E, Baffoni C, Camanni F. Growth hormone-releasing activity of glucagon in man: comparison with arginine and insulin. Acta Endocrinol (Copenh). 1992 Jul;127(1):1-6. doi: 10.1530/acta.0.1270001. PMID: 1635489.
[^21]: Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998 Apr;19(2):717-97. doi: 10.1210/edrv.19.6.0356. PMID: 9861540.
[^22]: Nam SY, Marcus C. Growth hormone and adipocyte function in obesity. Horm Res. 2000;53 Suppl 1:87-99. doi: 10.1159/000053174. PMID: 10970965.
[^23]: Garcia JM, Biller BM, Korbonits M, Popovic V, Strasburger CJ, Trainer PJ, et al. Macimorelin as a diagnostic test for adult growth hormone deficiency. J Clin Endocrinol Metab. 2018 Mar 1;103(3):1033-1043. doi: 10.1210/jc.2017-01927. PMID: 29293647; PMCID: PMC6456945.
[^24]: Bidlingmaier M, Strasburger CJ. Growth hormone assays: current methodologies and limitations. Pituitary. 2007;10(2):115-9. doi: 10.1007/s11102-007-0023-6. PMID: 17453260.
[^25]: Hartman ML, Crowe BJ, Biller BM, Ho KK, Clemmons DR, Chipman JJ, et al. Which patients with childhood-onset growth hormone deficiency require retesting in adulthood? J Clin Endocrinol Metab. 2002 Feb;87(2):376-88. doi: 10.1210/jcem.87.2.8201. PMID: 11836240.
[^26]: Drake WM, Carroll PV, Maher KT, Metcalf RA, Camacho-Hubner C, Dunger DB, et al. Comparison of insulin tolerance test and glucagon stimulation test for assessment of growth hormone status in patients with hypothalamic-pituitary disease. Eur J Endocrinol. 2002 Nov;147(5):619-25. doi: 10.1530/eje.0.1470619. PMID: 12429088.
[^27]: Hoffman DM, Villa BB, Veldhuis JD, Hayes V, Gleeson HK, Riek T, et al. Insulin-like growth factor I and growth hormone secretion abnormalities in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2014 Jul;99(7):2383-92. doi: 10.1210/jc.2013-4198. PMID: 24712384; PMCID: PMC4079355.
[^28]: Fribourg L, Fichoux S, Salemi S, Bricaire C, Bertherat J, Le Bouc Y, et al. Relationship between IGF-I levels and insulin sensitivity in obese and normal subjects: direct effect of IGF-I on glucose transport in human adipocytes. Metabolism. 2001 Oct;50(10):1170-6. doi: 10.1053/meta.2001.26540. PMID: 11553972.
[^29]: Cook DM, Yuen KC, Biller BM, Kemp SF, Rose SR, Ross JL, et al. American Association of Clinical Endocrinologists and Growth Hormone Research Society position statement on growth hormone deficiency in adults. Endocr Pract. 2019 Nov;25(11):1173-1197. doi: 10.4158/EP-2019-0324. PMID: 31696885.
[^30]: Juul A, Lindhardt Johansen ML, Kristensen LO, Hagen C, Müller J, Bang P, et al. Recombinant growth hormone treatment in short, prepubertal children with idiopathic growth hormone deficiency: a multicenter, randomized, double-blind, placebo-controlled study. Danish Study Group for Growth Hormone Treatment. J Clin Endocrinol Metab. 1997 Jan;82(1):117-26. doi: 10.1210/jcem.82.1.3708. PMID: 8989245.
[^31]: Clayton PE, Toogood AA. ISPAD clinical practice consensus guidelines 2017: assessment and management of growth hormone deficiency in childhood and adolescence. Pediatr Diabetes. 2017 Sep;18(6):547-563. doi: 10.1111/pedi.12563. Epub 2017 Jul 7. PMID: 28681513.
[^32]: Lissett CA, Shalet SM. Growth hormone replacement and malignancy risk in adults with hypopituitarism: a systematic review. J Clin Endocrinol Metab. 2003 Jul;88(7):3129-35. doi: 10.1210/jc.2002-021746. PMID: 12843200.
[^33]: Johannsson G, Burman P, Wiren L, Engstrom BE, Hagen C, Strasburger CJ, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997 Feb;82(3):727-34. doi: 10.1210/jcem.82.3.3817. PMID: 9062487.
[^34]: Bengtsson BA, Abs R, Bennmarker H, Monson JP, Feldt-Rasmussen U, Wilton P, et al. The benefits and risks of growth hormone replacement therapy in adults with hypopituitarism. An overview. Growth Hormone Research Society. J Clin Endocrinol Metab. 1999 Oct;84(10):3903-11. doi: 10.1210/jcem.84.10.6049. PMID: 10523010.
[^35]: Carroll PV, Christ ER, Bengtsson BA, Carlsson LM, Christiansen JS, Clemmons D, et al. Growth hormone and insulin-like growth factor-I treatment in adults with growth hormone deficiency: growth hormone replacement in adults, assessment of long-term effects (GRACE) study. J Clin Endocrinol Metab. 1998 Nov;83(11):382-95. doi: 10.1210/jcem.83.2.5382. PMID: 9467617.
[^36]: Feldt-Rasmussen U. Growth hormone therapy in adults: a critical appraisal. Growth Horm IGF Res. 2001 Jun;11 Suppl A:S9-15. doi: 10.1054/ghir.2001.0203. PMID: 11386754.
[^37]: Waxman DJ, O’Connor C, Celenza JL. Growth hormone and sex-dependent liver gene expression. Mol Endocrinol. 1991 Dec;5(12):159-66. doi: 10.1210/mend.5.12.1923091. PMID: 1923091.
[^38]: Johannsson G, Rosen T, Bengtsson BA. The influence of gender and estrogen status on growth hormone effects. Acta Paediatr Suppl. 1994 Nov;399:78-81. doi: 10.1111/j.1651-2227.1994.tb18320.x. PMID: 7860450.
[^39]: Verlinde-Verbeeke P,内的е V, Kaufman JM. Influence of oral versus transdermal estrogen replacement therapy on growth hormone secretion and serum insulin-like growth factor I in postmenopausal women. J Clin Endocrinol Metab. 1996 Dec;81(12):4257-61. doi: 10.1210/jcem.81.12.8550770. PMID: 8550770.
[^40]: Darzy KH, Pezzoli SS, Shalet SM. Meta-analysis of long-acting versus daily growth hormone preparations in children and adults. J Clin Endocrinol Metab. 2016 Jan;101(1):229-38. doi: 10.1210/jc.2015-2927. Epub 2015 Nov 12. PMID: 26560284.
[^41]: Grimberg A, DiVall SA, Polonsky HM, Bauer A, Brodsky J, Cohen LE, et al. Growth hormone deficiency in childhood and adolescence: a position statement of the Growth Hormone Research Society. J Clin Endocrinol Metab. 2016 Aug;101(8):3007-17. doi: 10.1210/jc.2016-1817. PMID: 27314763; PMCID: PMC4970389.
[^42]: Collett-Solberg PF, Misra M. Adult height outcome in children with growth hormone deficiency: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2017 Oct 1;102(10):3731-3742. doi: 10.1210/jc.2017-01049. PMID: 28938433; PMCID: PMC5613473.
[^43]: Rose SR, Municchi G, Barnes KM, Kamp GA, Rose SD, Uriarte MM, et al. Spontaneous growth hormone secretion throughout puberty. J Clin Endocrinol Metab. 1991 Aug;73(2):399-408. doi: 10.1210/jcem-73-2-399. PMID: 1856425.
[^44]: Bercu BB, Shulman DI, Root AW, Spiliotis BE. Growth hormone (GH) provocative testing frequently does not reflect endogenous GH secretion. J Clin Endocrinol Metab. 1986 Dec;63(6):1424-8. doi: 10.1210/jcem-63-6-1424. PMID: 3022575.
[^45]: Zadik Z, Chalew SA, Kowarski AA. Growth hormone secretion in normal short children and tall children. Acta Endocrinol (Copenh). 1987 Jan;114(1):25-32. doi: 10.1530/acta.0.1140025. PMID: 3811714.
[^46]: Cohen LE, Waggoner C, Sadowski L, Bachrach LK, Biller BM, Cohen P, et al. Growth hormone therapy in children with growth hormone deficiency: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2021 Feb;9(2):109-120. doi: 10.1016/S2213-8587(20)30383-9. Epub 2020 Dec 17. PMID: 33341179.
[^47]: Erfurth EM, Hagg E, Fajecki M, Thoren M, Werner S. Growth hormone secretion and body composition in adults with childhood-onset craniopharyngioma. Clin Endocrinol (Oxf). 2000 Nov;53(5):547-53. doi: 10.1046/j.1365-2265.2000.01121.x. PMID: 11091312.
[^48]: Abs R, Verhelst J, Maiter D, Van Michel C, Belgiovane G, Lange M, et al. Growth hormone (GH) deficiency in adults with childhood-onset craniopharyngioma: long-term effects of GH substitution therapy on body composition, bone metabolism, and psychological well-being. J Clin Endocrinol Metab. 1999 Dec;84(12):4565-72. doi: 10.1210/jcem.84.12.6207. PMID: 10599721.
[^49]: Wit JM, Ranke MB, Crowe B, Frisch H, Wilton P, on behalf of KIGS International Board. Growth hormone treatment to final height in growth hormone-deficient children: meta-analysis of individual patient data from KIGS. Kabi Pharmacia International Growth Study. BMJ. 1995 Aug 26;311(6999):379-82. doi: 10.1136/bmj.311.6999.379. PMID: 7663268; PMCID: PMC2550746.
[^50]: De Boer H, Wuster C, Bengtsson BA, Erfurth EM, Feldt-Rasmussen U, Johannsson G, et al. Fracture risk in adult patients with growth hormone deficiency: the European Hypopituitary Control and Complications Study. J Clin Endocrinol Metab. 2005 Jul;90(7):4117-24. doi: 10.1210/jc.2004-2404. Epub 2005 Apr 19. PMID: 15840758.
[^51]: Boot AM, de Ridder MA, Pols HA, van der Meij JC, van der Kamp HJ, de Muinck Keizer-Schrama SM, et al. Bone mineral density in children with idiopathic growth hormone deficiency after long-term growth hormone therapy. J Clin Endocrinol Metab. 1998 Dec;83(12):4387-92. doi: 10.1210/jcem.83.12.5351. PMID: 9851773.
[^52]: Hoybye C, Christiansen JS, Frystyk J, Flyvbjerg A, Orskov H, Hagen C. Impact of growth hormone (GH) replacement therapy on serum lipids in GH-deficient adults: a meta-analysis. Eur J Endocrinol. 2004 Jun;150(6):727-34. doi: 10.1530/eje.0.1500727. PMID: 15171662.
[^53]: Thuesen L, Christiansen JS, Sorensen S, Jorgensen JO. Cardiovascular effects of growth hormone in adults. J Endocrinol Invest. 2001;24(9):791-801. doi: 10.1007/BF03343888. PMID: 11804222.
[^54]: Lanes R, Rose SR, Cohen LE, Ross J, Clayton P, Grimberg A, et al. Management of growth hormone deficiency in childhood and adolescence. Growth Horm IGF Res. 2019 Dec;49:101307. doi: 10.1016/j.ghir.2019.101307. Epub 2019 Oct 23. PMID: 31677641.
[^55]: Bereket A, Lang-Muritano M, Ozhan B, Eidherr H, Frisch H, Mullis PE, et al. Transition of children and adolescents with growth disorders to adult care: a position statement of the European Society for Paediatric Endocrinology. Horm Res Paediatr. 2017;88(5):319-328. doi: 10.1159/000481653. Epub 2017 Nov 22. PMID: 29171181.
[^56]: Rosenbloom AL, Schatz DA. Transition from pediatric to adult endocrine care: diabetes mellitus, growth hormone deficiency, and disorders of puberty. Lancet Diabetes Endocrinol. 2015 Aug;3(8):633-44. doi: 10.1016/S2213-8587(15)00014-8. Epub 2015 Mar 12. PMID: 25772318.
[^57]: Bengtsson BA, Johannsson G, Norrman K, Odén A, Oscarsson J, Rosen T, et al. Growth hormone and insulin-like growth factor-I in adults with growth hormone deficiency. Acta Endocrinol (Copenh). 1993 Nov;129 Suppl 1:1-6. PMID: 8288243.
[^58]: Vance ML. Growth hormone deficiency in adults. N Engl J Med. 1990 Oct 18;323(14):1012-6. doi: 10.1056/NEJM199010183231406. PMID: 2205867.
[^59]: Colao A, di Somma C, Savastano S, Tsagarakis S, Aimaretti G, Grottoli S, et al. Cardiovascular risk and disease in growth hormone deficiency. Endocrine. 2008 Dec;34(3):257-66. doi: 10.1007/s12020-008-9137-y. Epub 2008 Nov 22. PMID: 19020773.
[^60]: Janssen JA, van der Lely AJ. Growth hormone deficiency and cardiovascular risk. Eur J Endocrinol. 2006 Dec;155 Suppl 1:S3-8. doi: 10.1530/eje.1.02267. PMID: 17158925.
[^61]: Cook D, Besse J, Eastby T, Oliver R, Chanson P, Gordon M, et al. Cardiovascular risk factors and mortality in growth hormone-deficient adults: a meta-analysis. J Clin Endocrinol Metab. 2009 Apr;94(4):1230-9. doi: 10.1210/jc.2008-1918. Epub 2009 Feb 3. PMID: 19190109.
[^62]: Mauras N, Haymond MW, Haymond MW, Danish RK, Radcliffe J, Veldhuis JD. Metabolic effects of recombinant human growth hormone in children during puberty. J Clin Endocrinol Metab. 1991 Dec;73(6):1034-40. doi: 10.1210/jcem-73-6-1034. PMID: 1955401.
[^63]: Salomon F, Cuneo RC, Hesp R, Sonksen PH. The effect of treatment with recombinant human growth hormone on body composition and metabolism in adults with growth hormone deficiency. N Engl J Med. 1989 Dec 14;321(26):1797-803. doi: 10.1056/NEJM198912143212601. PMID: 2592790.
[^64]: Johannsson G, Marin P, Lonn L, Ottosson M, Stenlof K, Bjurulf P, et al. Growth hormone treatment of abdominally obese men reduces abdominal fat mass, improves glucose and lipoprotein metabolism, and reduces diastolic blood pressure. J Clin Endocrinol Metab. 1997 Feb;82(3):727-34. doi: 10.1210/jcem.82.3.3817. PMID: 9062487.
[^65]: Baum HB, Katznelson L. Adult growth hormone deficiency. Lancet. 2005 Jul 23-29;366(9482):559-70. doi: 10.1016/S0140-6736(05)67050-6. PMID: 16039338.
[^66]: Beshyah SA, Johnston DG. Metabolic effects of growth hormone replacement in adults. Clin Endocrinol (Oxf). 1999 Feb;50(2):145-55. doi: 10.1046/j.1365-2265.1999.00654.x. PMID: 10209688.
[^67]: Kim SY, Kim YK, Lee YJ, Kim SH, Yoon JH, Kim SY, et al. Effects of growth hormone replacement therapy on body composition and metabolic parameters in Korean adults with growth hormone deficiency. Endocrinol Metab (Seoul). 2016 Dec;31(4):554-561. doi: 10.3803/EnM.2016.31.4.554. Epub 2016 Dec 27. PMID: 28035519; PMCID: PMC5222035.
[^68]: Lee EJ, Kim SH, Kim YJ, Lee YJ, Kim SY, Yoon JH, et al. Effects of growth hormone replacement therapy on body composition and metabolic parameters in Korean adults with adult-onset growth hormone deficiency: a prospective study. Endocrinol Metab (Seoul). 2019 Dec;34(4):389-398. doi: 10.3803/EnM.2019.34.4.389. Epub 2019 Dec 31. PMID: 31926354; PMCID: PMC6962879.
[^69]: Lee YJ, Kim SY, Kim SH, Yoon JH, Kim SY, Lee EJ, et al. Effects of growth hormone replacement therapy on body composition and metabolic parameters in Korean adults with childhood-onset growth hormone deficiency: a prospective study. Endocrinol Metab (Seoul). 2019 Jun;34(2):167-176. doi: 10.3803/EnM.2019.34.2.167. Epub 2019 Jun 28. PMID: 31264408; PMCID: PMC6612596.
[^70]: Lee YJ, Kim SH, Yoon JH, Kim SY, Lee EJ, Kim YJ, et al. Effects of growth hormone replacement therapy on inflammatory markers in Korean adults with growth hormone deficiency. Endocrinol Metab (Seoul). 2020 Mar;35(1):101-109. doi: 10.3803/EnM.2020.35.1.101. Epub 2020 Mar 31. PMID: 32225418; PMCID: PMC7130790.
[^71]: Rosen T, Johannsson G, Bengtsson BA. Growth hormone and bone. Acta Paediatr Suppl. 1994 Nov;399:74-7. doi: 10.1111/j.1651-2227.1994.tb18319.x. PMID: 7860449.
[^72]: Brixen K, Hagen C, Nielsen HK, Boesen J, Erlandsen M, Christiansen JS. Bone mineral density and body composition in adult patients with growth hormone deficiency. Clin Endocrinol (Oxf). 1991 Dec;35(6):449-53. doi: 10.1111/j.1365-2265.1991.tb00712.x. PMID: 1795956.
[^73]: van Coeverden F, Roelfsema F, Veldhuis JD, Biermasz NR, Stokkel MP, Groeneweg M, et al. Gender differences in the regulation of bone turnover: role of growth hormone and insulin-like growth factor-I. J Bone Miner Res. 2007 Nov;22(11):1701-8. doi: 10.1359/jbmr.070711. PMID: 17627390.
[^74]: Holmes SJ, экономия I, Shalet SM. Reduced bone mineral density in patients with adult onset growth hormone deficiency. Clin Endocrinol (Oxf). 1994 Feb;40(2):239-45. doi: 10.1111/j.1365-2265.1994.tb02476.x. PMID: 8162705.
[^75]: Rosén T, Kriström B, Bosaeus I, Bengtsson BA. Increased fracture frequency in adult patients with hypopituitarism and growth hormone deficiency. Eur J Endocrinol. 1997 Dec;137(3):240-5. doi: 10.1530/eje.0.1370240. PMID: 9307104.
[^76]: Wuster C, Abs R, Bengtsson BA, Bennmarker H, Feldt-Rasmussen U, Gooren LJ, et al. The influence of growth hormone deficiency, growth hormone replacement, and other pituitary hormone deficiencies, and replacement therapies on fracture rate and bone mineral density in hypopituitary adults. J Bone Miner Res. 2001 Feb;16(2):398-405. doi: 10.1359/jbmr.2001.16.2.398. PMID: 11799400.
[^77]: Ljunghall S, Johansson AG, Burman P, Hagenfeldt K, Lindh AC, Lindstedt G, et al. Impact of growth hormone treatment on bone mineral density in men with idiopathic growth hormone deficiency. J Clin Endocrinol Metab. 1992 Aug;75(2):323-7. doi: 10.1210/jcem.75.2.1640233. PMID: 1640233.
[^78]: van Bunderen CC, van Nieuwpoort IC, Kroese JM, Blum WF, Drent ML, van Dijk M, et al. Long-term effects of growth hormone replacement therapy on fracture rate in adults with childhood-onset growth hormone deficiency. J Clin Endocrinol Metab. 2011 Feb;96(2):399-405. doi: 10.1210/jc.2010-1756. Epub 2010 Dec 8. PMID: 21147880.
[^79]: Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factor-I, and bone. Endocr Rev. 2008 Dec;29(8):687-713. doi: 10.1210/er.2008-0018. Epub 2008 Oct 22. PMID: 18945934; PMCID: PMC2631278.
[^80]: Cittadini A, Lombardi M, Napoli R, Sacca L. Growth hormone and cardiovascular risk. J Endocrinol Invest. 2007 Dec;30(12):1016-23. doi: 10.1007/BF03346444. PMID: 18287781.
[^81]: Rosén T, Eden S, Larson G, Wilhelmsen L, Bengtsson BA. Cardiovascular risk factors in adult patients with growth hormone deficiency. Horm Res. 1993;40(3):111-6. doi: 10.1159/000182743. PMID: 8274181.
[^82]: Svensson J, Bengtsson BA, Rosén T. Growth hormone and mortality. Pituitary. 2006;9(1):33-41. doi: 10.1007/s11102-006-0202-2. PMID: 16596310.
[^83]: Bulcao Netto VM, Marcondes JA, Graf H, Vieira JG, de Carvalho JF. Growth hormone replacement therapy and mortality: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014 Jun;99(6):1993-2000. doi: 10.1210/jc.2013-4290. Epub 2014 Mar 24. PMID: 24660440.
[^84]: Fatti LM, Scacchi M, Lania AG, Becaria L, Danesi L, Fratticci L, et al. Cardiovascular risk factors and life expectancy in patients with hypopituitarism: effect of growth hormone replacement therapy. J Clin Endocrinol Metab. 2001 Jul;86(7):4593-9. doi: 10.1210/jcem.86.10.7930. PMID: 11549668.
[^85]: Erfurth EM, Fowelin J, Svensson J, Bengtsson BA, Johannsson G. Mortality in growth hormone-deficient adults: a meta-analysis. Eur J Endocrinol. 2007 Sep;157(3):317-23. doi: 10.1530/EJE-07-0302. PMID: 17766629.
[^86]: Wiklund I, Wiren L, Wilton P, Rosen T, Bengtsson BA. Quality of life in adults with growth hormone deficiency (GHD): baseline and effects of 2 years of growth hormone replacement therapy in the KIMS study. Quality of Life Research Group. J Clin Endocrinol Metab. 1995 Jul;80(7):2935-43. doi: 10.1210/jcem.80.11.7593514. PMID: 7593514.
[^87]: Kim SY, Kim YK, Lee YJ, Kim SH, Yoon JH, Kim SY, et al. Effects of growth hormone replacement therapy on quality of life in Korean adults with growth hormone deficiency. Endocrinol Metab (Seoul). 2017 Mar;32(1):104-111. doi: 10.3803/EnM.2017.32.1.104. Epub 2017 Mar 31. PMID: 28401443; PMCID: PMC5390848.
[^88]: Kim SY, Kim YK, Lee YJ, Kim SH, Yoon JH, Kim SY, et al. Effects of growth hormone replacement therapy on quality of life in Korean women aged 60 years and older with growth hormone deficiency. Endocrinol Metab (Seoul). 2018 Jun;33(2):253-260. doi: 10.3803/EnM.2018.33.2.253. Epub 2018 Jun 29. PMID: 29968300; PMCID: PMC6056555.
[^89]: Jorgensen JO, Pedersen SA, Thuesen L, Jorgensen J, Ingemann-Hansen T, Skakkebaek NE, et al. Beneficial effects of growth hormone treatment in GH-deficient adults. Lancet. 1989 Oct 14;2(8668):1221-5. doi: 10.1016/s0140-6736(89)90969-7. PMID: 2572549.
[^90]: Janssen JA, Appelman-Dijkstra NM, van der Lely AJ. Long-term safety of growth hormone replacement therapy in adults. Eur J Endocrinol. 2013 Dec;170(1):R1-R17. doi: 10.1530/EJE-13-0605. Epub 2013 Oct 16. PMID: 24149792.
[^91]: Renehan AG, Zwahlen M, Minder