Intracranial calcifications (IC) are frequently identified on non-contrast computed tomography (NCCT) scans in both adults and children. These calcifications, occurring within the brain parenchyma or vasculature, are categorized into physiologic/age-related, dystrophic, congenital disorders/phakomatoses, infectious, vascular, neoplastic, metabolic/endocrine, inflammatory, and toxic etiologies. This updated review provides a comprehensive overview of intracranial calcifications across pediatric and adult populations, emphasizing their patterns, sizes, and locations to aid in radiological differential diagnosis.
Introduction
Intracranial calcifications, the deposition of calcium salts within the brain tissue or blood vessels (1), are common findings in neuroimaging. Their prevalence varies with age, from approximately 1% in younger individuals to as high as 20% in the elderly. Autopsy studies, including microscopic examinations, have reported even higher rates, reaching up to 72% (2).
Computed Tomography (CT) scanning has become indispensable in the detection, precise localization, and classification of intracranial calcifications (3). Despite advancements in Magnetic Resonance Imaging (MRI), CT remains the superior modality for identifying and characterizing brain calcifications due to its high sensitivity to calcium. Intracranial calcifications encompass both physiologic, age-related processes and a wide range of pathological conditions (2). Recognizing the radiological phenotypes of various intracranial calcifications is crucial, as they can be indicative of specific underlying pathologies, such as vascular calcifications in stroke or basal ganglia calcifications in hypoparathyroidism (1).
This review aims to present a broad spectrum of intracranial calcifications (Table 1 & 2), focusing on their radiological appearance, size, and anatomical location. This approach is intended to improve the clinical understanding and radiological differential diagnosis of intracranial calcifications (Figure 1).
Table 1.
Summary of intracranial calcifications
Table 2.
Detailed summary of intracranial calcifications with etiologies and radiological features for differential diagnosis.
| Intracranial Calcification | Etiologies | Location/Pattern |
|---|---|---|
| Physiologic/Age-Related Intracranial Calcifications | Normal aging process | Pineal gland, choroid plexus, habenula, falx cerebri, tentorium cerebelli, and basal ganglia. Appear coarse and compact, punctate, or curvilinear. |
| Genetic / Developmental Disorders | Inherited conditions and developmental anomalies | |
| Sturge-Weber syndrome | Sporadic neurocutaneous disorder | Tram track appearance, double-lined gyriform pattern, reflecting cortical calcifications. |
| Tuberous sclerosis | Autosomal dominant genetic disorder | Subcortical and subependymal tubers, often calcified, located along the caudothalamic groove and atrium. |
| Neurofibromatosis | Genetic disorders affecting nerve tissue growth | Calcifications of the choroid plexus of the lateral ventricles and nodular calcifications of the cerebellum. |
| Cockayne syndrome | Autosomal recessive progeroid syndrome | Bilateral rock or spot calcifications at the level of the basal ganglia, sometimes with gyral calcifications. |
| Krabbe disease | Autosomal recessive leukodystrophy | Internal capsule and corona radiata, affecting white matter tracts. |
| Aicardi-Goutières Syndrome | Autosomal recessive encephalopathy | Symmetrical, spot-like calcifications at the level of the basal ganglia and deep white matter of both frontal and parietal lobes. |
| Fahr disease | Rare neurodegenerative condition | Symmetrical, involving the caudate, putamen, globus pallidus, thalamus, deep cortex, and dentate nuclei. |
| Congenital Infection | Infections acquired in utero | |
| Cytomegalovirus (CMV) | Common congenital viral infection | Thick chunky periventricular calcifications and faint punctate calcifications in the basal ganglia. |
| Herpes Simplex Virus (HSV) | Less common but severe neonatal infection | Scattered, reflecting widespread brain damage. |
| Toxoplasmosis | Parasitic infection transplacentally acquired | Nodular periventricular and cortical calcifications, curvilinear in the thalamus and basal ganglia. |
| Rubella | Viral infection, now rare due to vaccination | Basal ganglia and periventricular area calcifications. |
| Zika Virus | Emerging viral infection | Punctate calcifications located between the cortex and subcortical white matter. |
| Human Immunodeficiency Virus (HIV) | Vertical transmission of HIV | Symmetrical calcifications in the basal ganglia and subcortical matter. |
| Acquired infection | Infections acquired postnatally | |
| Neurocysticercosis | Parasitic infection from Taenia solium | Eccentric calcified nodule within a peripherally calcified cyst in the brain parenchyma and subarachnoid space. |
| Mycobacterium tuberculosis | Bacterial infection, causing tuberculosis | Tuberculomas may calcify centrally, creating a pathognomonic target sign. |
| Cryptococcus neoformans | Fungal infection, especially in immunocompromised individuals | Punctate calcifications in the brain parenchyma and leptomeninges. |
| Vascular | Related to blood vessels | |
| Intracranial Atherosclerosis | Arterial plaque buildup | Scattered dots or stippled, symmetrical subcortical calcifications within vessel walls. |
| Intra-axial Neoplastic | Tumors within the brain parenchyma | |
| Astrocytoma (various types) | Primary brain tumor from astrocytes | Heterogeneous calcifications, variable patterns. |
| Oligodendroglioma | Primary brain tumor from oligodendrocytes | Nodular and clumped calcifications. |
| Ganglioglioma | Tumor containing neuronal and glial cells | Mural calcified nodule within the tumor. |
| Medulloblastoma | Malignant pediatric cerebellar tumor | Tiny scattered dots or clumped calcifications. |
| Extra-axial Neoplastic | Tumors outside the brain parenchyma | |
| Meningioma | Tumor arising from the meninges | Sand-like, sunburst, rim, and globular calcifications. |
| Craniopharyngioma | Tumor near the pituitary gland | Thin and circumferential or chunky calcifications, often cystic. |
| Pineal tumors (various types) | Tumors of the pineal gland | Peripheral exploded pattern of calcifications. |
| Germ cell tumors | Tumors from germ cells | Heterogeneous calcifications. |
| Lipoma | Benign fatty tumor | Egg-shell or central calcifications. |
| Intraventricular Neoplastic | Tumors within the ventricles | |
| Ependymoma | Tumor arising from ependymal cells lining ventricles | Dots or mass/rock-like calcifications. |
| Central neurocytoma | Benign tumor within the ventricles | Dots to large masses of calcifications. |
| Metabolic/Endocrine | Metabolic or hormonal imbalances | |
| Hypoparathyroidism | Deficiency of parathyroid hormone | Basal ganglia, predominantly globus pallidus, symmetrical calcifications. |
| Inflammatory | Inflammatory conditions affecting the brain | |
| Systemic lupus erythematosus (SLE) | Autoimmune disease | Cerebellum mostly, but can involve other areas, symmetrical calcifications. |
| Sarcoidosis | Inflammatory disease with granuloma formation | Suprasellar, hypothalamic, and cerebellar areas. |
| Neurotoxicity | Exposure to toxic substances | |
| Lead and carbon monoxide poisoning | Environmental toxins | Punctiform, curvilinear, speck-like, and diffuse calcifications. |
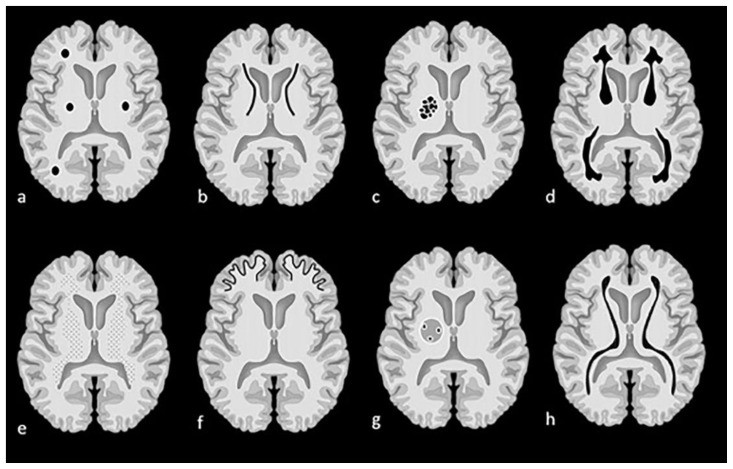
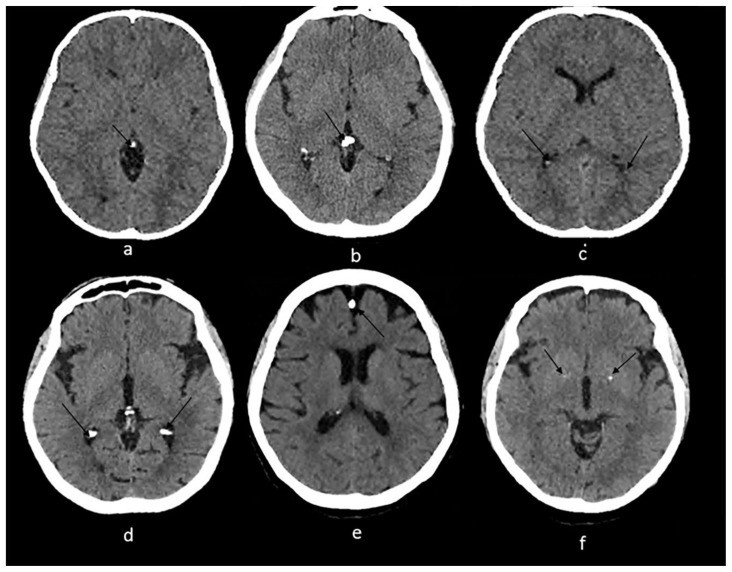
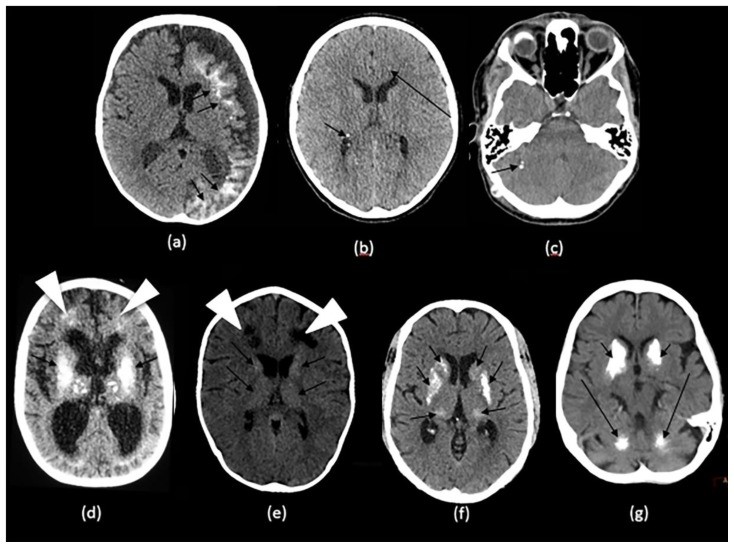
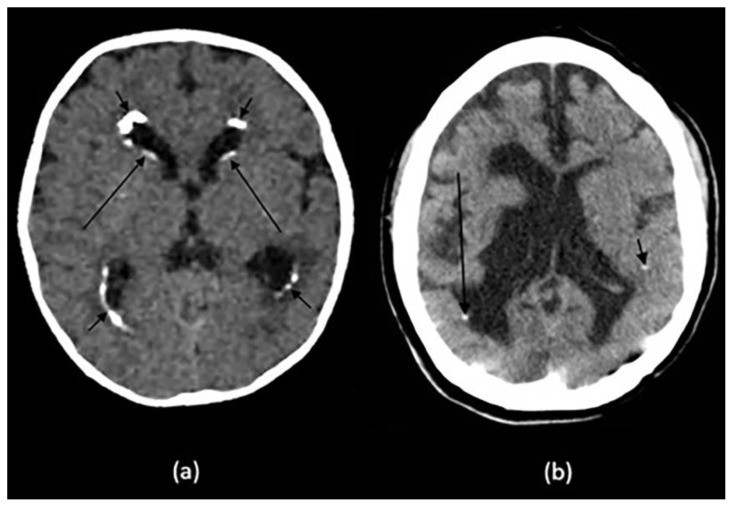
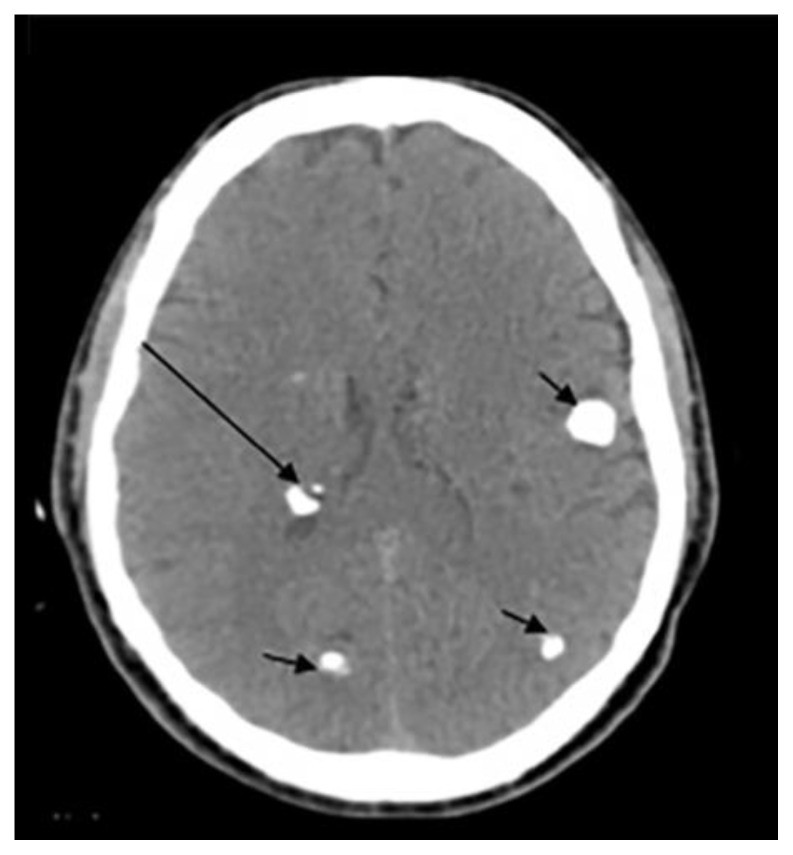
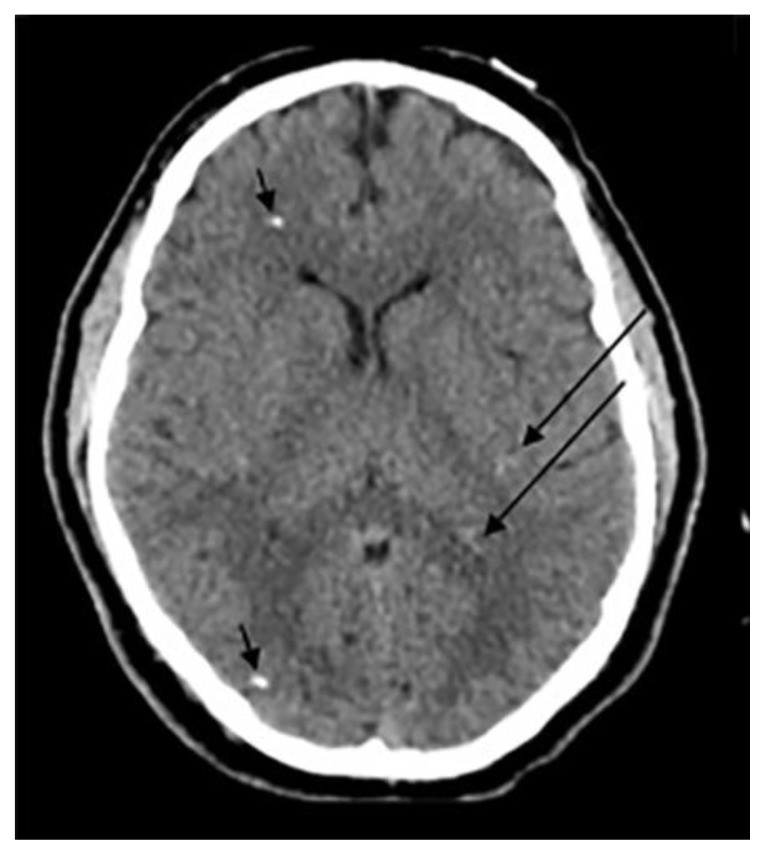
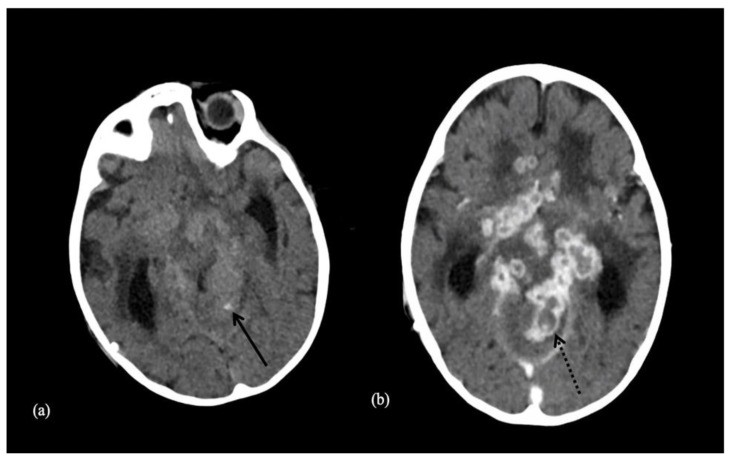
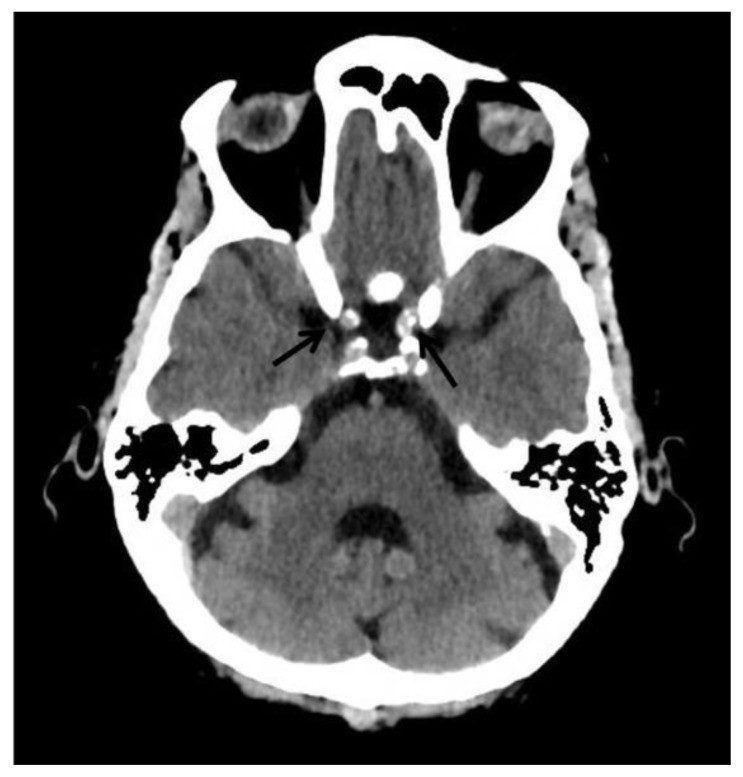
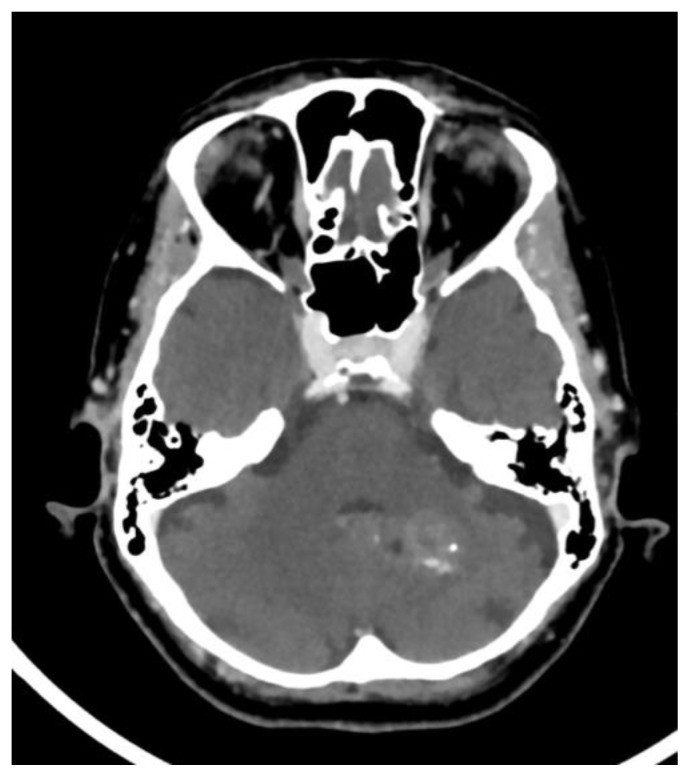
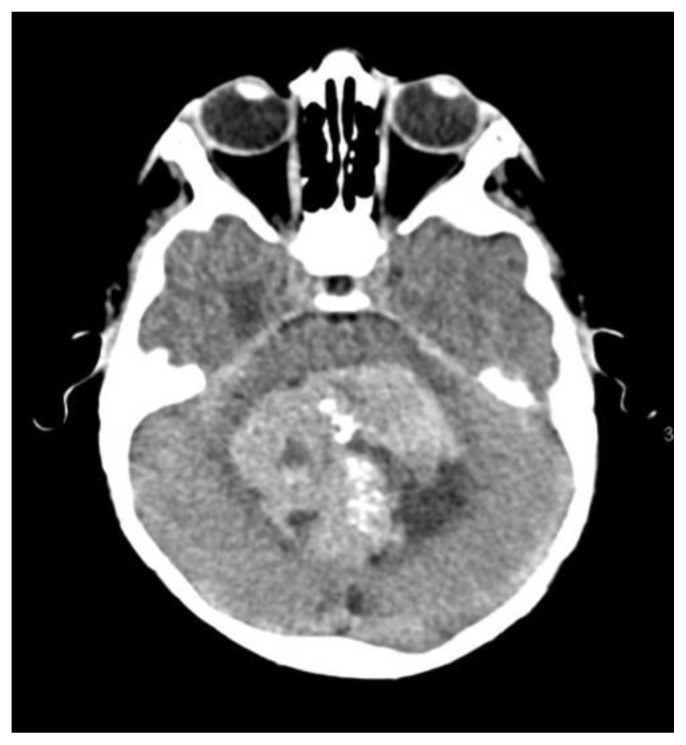
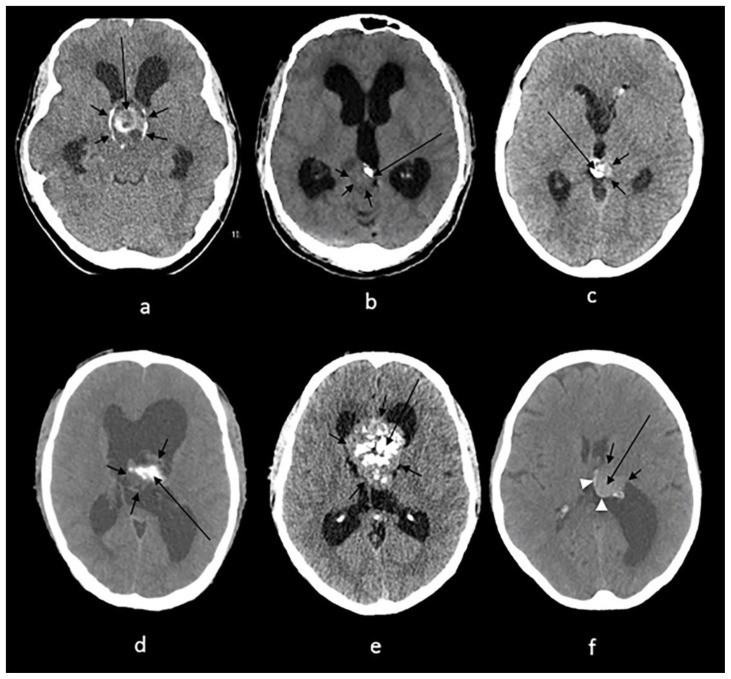
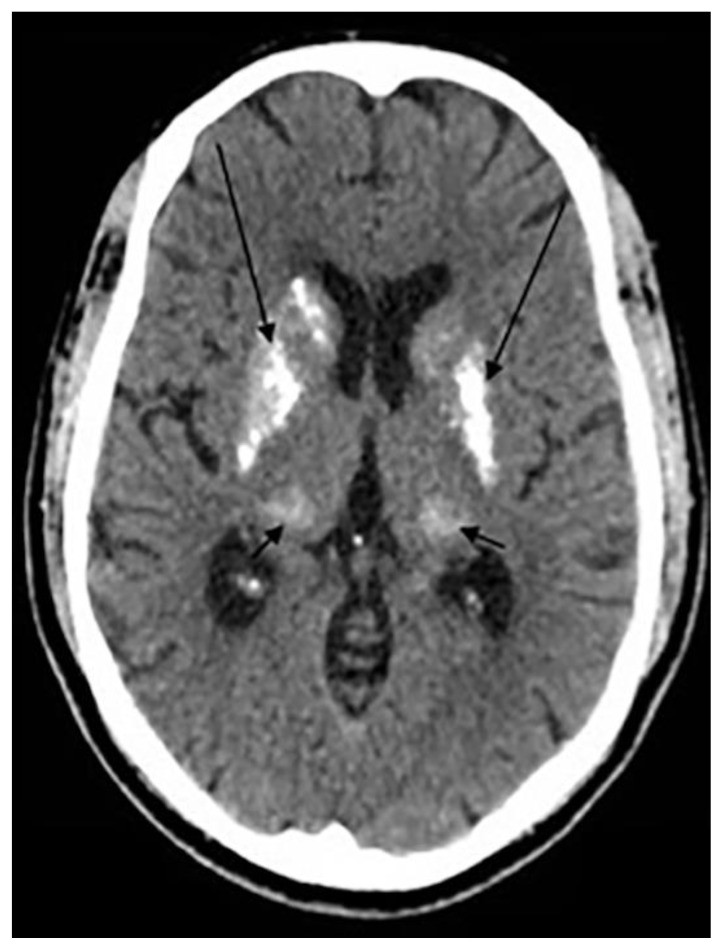
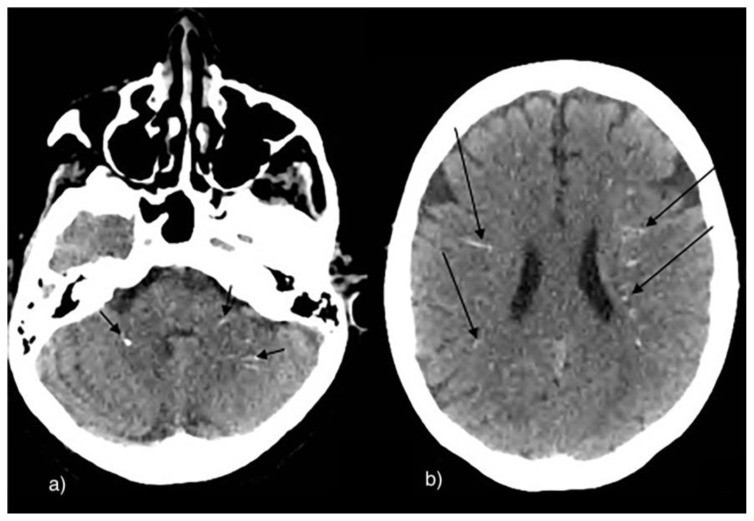
Figure 1.
Examples of intracranial calcification patterns and terminology relevant to radiology differential diagnosis.
Examples of patterns of calcification and related terminology. (a) dots, (b) lines, (c) conglomerate or mass-like, (d) rock-like, (e) blush, (f) gyriform/band-like, (g) stippled (h) reticular.
Physiologic/Age-Related Intracranial Calcifications
Physiologic intracranial calcifications are common incidental findings on NCCT scans, becoming more prevalent with advancing age. These calcifications are not associated with any underlying pathology and are considered a normal part of the aging process.
Common locations for physiologic calcifications include the pineal gland, choroid plexus, habenula, falx cerebri, and tentorium cerebelli (4, 5). A large study by Yalcin et al., involving 11,941 subjects, found the pineal gland to be the most frequent site (71.6%), followed by the choroid plexus (70.2%). Both sites showed a male predominance, with mean ages of 47.3 and 49.8 years, respectively. Notably, choroid plexus calcifications became the most common physiologic type after the fifth decade and second most common after pineal gland calcifications in individuals aged 15–45 years (3). In contrast, a study by Whitehead et al. on 500 pediatric subjects revealed physiologic calcifications in 40% (202/500), with 97% being older than 5 years. In children, choroid plexus was the most common site (58/500), followed by habenula (50/500) and pineal gland (25/500) (5).
Pineal calcifications typically appear coarse and compact (6) (Fig. 2a and 2b). Whitehead et al. observed that in children younger than 7 years, pineal calcifications were punctate and solitary, occasionally becoming larger and more numerous after age 7. This suggests that large or multiple pineal calcifications in young children (<7 years) warrant careful evaluation for potential underlying neoplasms (5). Other studies suggest further investigation for calcifications exceeding 1 cm or in patients younger than 9 years, as these may indicate neoplasms (4, 6). Suspicious pineal calcifications should be assessed further with glandular volume measurement, clinical correlation, and biochemical investigations.
Figure 2.
Radiological examples of physiologic intracranial calcifications on axial NCCT.
Technique: Axial non enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
(a): 55-year-old female presenting to the ER post trauma.
Findings: Incidental dots of calcifications in the pineal gland (arrow).
(b) and (c): An 11-year-old male who presented to ER post MVA.
Findings: Incidental tiny dots (less than 1 cm) of calcifications with no soft tissue component in the pineal gland (2b) and choroid plexuses (2c) (arrows), a pattern suggestive of benign/ physiologic nature.
(d): 66-year-old male who presented to the ER for dizziness.
Findings: Incidental dots of calcifications in the bilateral choroid plexuses (arrows).
(e): 73-year-old male presenting to ER for high blood pressure and headache.
Findings: Incidental dot of calcification in anterior cerebral falx (arrow).
(f): 79-year-old male who presented to the ER post trauma.
Findings: Incidental isolated dots of basal ganglia calcifications (arrows).
Choroid plexus calcifications typically occur in the atria of the lateral ventricles and are less common in the third and fourth ventricles or in children under 9 years (6) (Fig. 2c and 2d). In Whitehead et al.’s pediatric study, all choroid plexus calcifications were in the lateral ventricles and mostly punctate (5). However, atypical locations such as the glomerula, bodies of the lateral ventricles, roof of the third ventricle, and foramina of Monro should raise suspicion for underlying pathology (4).
Habenular calcifications often present with a curvilinear pattern anterior to the pineal body, observed in up to 15% of adults (4, 6). The habenula’s connections to deep brain areas, influencing dopamine and serotonin, have linked habenular calcifications to schizophrenia (5).
Dural calcifications, including falx cerebri and tentorium cerebelli, were found in 12.5% of Yalcin et al.’s study population, predominantly in older males (mean age 53.1). Notably, choroid plexus calcifications co-occurred in 78.4% of patients with dural calcifications (3). Whitehead et al. found dural calcifications in only 1% of children (age range 2.9–8.7 years), mostly in the tentorium and falx cerebri (5) (Fig. 2e).
Basal ganglia calcifications (BGC) were the least common physiologic calcification in Yalcin et al.’s study (1.3%), more prevalent in females (mean age 52.4), and their prevalence did not increase with age. Most BGC were in the globus pallidus and co-occurred with pineal calcifications in 82.6% of cases (3) (Fig. 2f).
Genetic / Developmental Disorders
Intracranial calcifications are frequently observed in neurocutaneous syndromes, particularly Sturge-Weber syndrome and tuberous sclerosis, but less commonly in neurofibromatosis and Cockayne syndrome (CS) (4, 6).
Sturge-Weber syndrome (SWS), or encephalotrigeminal angiomatosis, is a neurocutaneous syndrome characterized by a facial port-wine stain, ipsilateral leptomeningeal vascular malformation, and intracranial calcifications. Neurological symptoms include seizures, hemiparesis, visual field deficits, and intellectual disability (7, 8). NCCT typically reveals “tram-track” or double-lined gyriform calcifications parallel to cerebral convolutions, resulting from cortical or subcortical ischemia due to pial angiomatosis (1). However, these calcifications may not be evident in the first year of life and can take several years to become detectable (8) (Fig. 3a).
Figure 3.
NCCT examples of intracranial calcifications in genetic and developmental disorders affecting children and adults.
Technique: Axial non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness
(a): 1-month-old infant boy with Sturge-Webber syndrome.
Findings: Diffuse gyral/subcortical white matter calcifications (arrows) with volume loss in the left cerebral hemisphere.
(b): 6-year-old boy with tuberous sclerosis.
Findings: Subependymal (short arrows) and subcoritcal tubers (long arrows), some of which are calcified.
(c): 40-year-old female with NF 2.
Findings: Right cerebellar hemisphere calcifications (arrow).
(d): 29-year- old male with Cockayne disease.
Findings: Bilateral conglomerate of calcifications in the globus pallidi, putamina, caudate nuclei and thalami (arrows) as well in the subcortical white matter (arrowheads). There is cerebral volume loss as seen by prominence of sulci and ventricles.
(e): 4-year-old boy with Krabbe s disease.
Findings: Blush-like calcifications within the bilateral basal ganglia (arrows) including the thalami early in the course of the disease. In addition, there is decreased bifrontal peri-ventricular density from demyelination (arrowheads).
(f) 3-month-old infant girl with AGS.
Findings: Conglomerate of intracranial calcifications almost equally involving the basal ganglia (arrows) including the thalami. There is slight ex-vacuo dilatation of the ventricles and slight prominence of cerebral sulci secondary to volume loss.
(g): 42-year-old man with Fahr disease.
Findings: Conglomerate/mass like calcifications in the bilateral basal ganglia (short arrows), bilateral cerebellar white matter and dentate nuclei (long arrows).
Tuberous sclerosis (TS), an autosomal dominant disorder, causes hamartomas in various organs, including the brain. Neuroimaging shows subependymal hamartomas, subcortical tubers, giant cell tumors, and white matter lesions. Intracranial calcifications occur in up to 54% of TS patients and are more common with age, with calcified subcortical tubers being more prevalent in older individuals. Calcification of subependymal nodules, typically along the caudothalamic groove and atrium, is pathognomonic (6, 9) (Fig. 3b).
Neurofibromatosis (NF), a group of hereditary cancer syndromes, leads to tumors of the central and peripheral nervous systems. NF type 1 (NF1) is more common than NF type 2 (NF2). Tumors in NF1 include nerve sheath tumors, gliomas, and others. NF2 is characterized by schwannomas, meningiomas, ependymomas, and ocular abnormalities (10). Intracranial calcifications in NF result from calcium deposition in hamartomatous glial proliferations, similar to TS (1, 4). Non-tumoral calcifications are typically symmetrical or asymmetrical in the choroid plexus of the lateral ventricles and nodular in the cerebellum. In NF2, calcifications are often associated with tumors, particularly meningiomas (4) (Fig. 3c).
Cockayne syndrome (CS), an autosomal recessive disorder, is characterized by dwarfism, skin abnormalities, and photosensitivity. Brain calcifications are common in CS patients older than 3 years, but rare before age 1, even in severe cases (11). Calcifications are typically bilateral, rock-like or spot-like in the basal ganglia, sometimes with gyral involvement. Cerebellar, dentate, thalamic, and leptomeningeal calcifications are less frequently observed (1, 11) (Fig.3d).
Krabbe disease, an autosomal recessive leukodystrophy, presents early in infancy (before 4 months) with irritability, hypersensitivity, startle response, spasticity, feeding difficulties, fever, and microcephaly (12). Brain calcifications are noted in the internal capsule and corona radiata, regions of abnormal white matter and globoid cell accumulation (1) (Fig. 3e).
Aicardi-Goutières Syndrome (AGS) is an autosomal recessive encephalopathy presenting neonatally or in infancy. Neonatal AGS involves fever, seizures, hepatosplenomegaly, thrombocytopenia, and anemia, with or without microcephaly. Infantile AGS presents with irritability, fever, loss of skills, and acquired microcephaly. Brain calcifications in AGS are symmetrical, spot-like in the basal ganglia and deep white matter of the frontal and parietal lobes. Other affected sites include the dentate nucleus, cerebellar cortex, brainstem, and cerebral cortex (1) (Fig. 3f).
Fahr disease, a rare neurodegenerative condition also known as idiopathic basal ganglia calcification, presents with varied clinical symptoms, from asymptomatic to severe movement and neuropsychological disorders (1, 13). Intracranial calcifications in Fahr disease primarily affect grey matter structures, and to a lesser extent white matter. They are typically symmetrical, involving the caudate, putamen, globus pallidus, thalamus, deep cortex, and dentate nuclei (1) (Fig. 3g).
Congenital/Acquired Infections
Congenital
Congenital Cytomegalovirus (CMV) infection is the most common TORCH infection, with a prevalence of 0.6–0.7% in industrialized countries. It results from transplacental transmission following maternal infection (14). Congenital CMV often presents with chorioretinitis, microcephaly, and intracranial calcifications (6). Intracranial calcifications are the most frequent neuroimaging finding, occurring in 34–70% of cases, and are strongly associated with developmental delay and intellectual disability. Calcifications typically occur in the periventricular area, brain parenchyma, or basal ganglia. Periventricular calcifications are described as thick and chunky, while basal ganglia calcifications are faint and punctate (14) (Fig. 4a).
Figure 4.
NCCT examples of intracranial calcifications due to congenital infections in infants and children.
Technique: Axial non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
(a): 10-month-old infant boy with seizures and neonatal CMV infection.
Findings: Reticular calcification pattern in the subependymal (long arrows) and periventricular region (short arrows) with mild ventriculomegaly.
(b): 21-year-old female with congenital toxoplasma infection.
Findings: Dots of calcification in the periventricular (long arrow) and subcortical (short arrow) regions with brain destruction, volume loss and ex-vacuo dilatation of the ventricles.
Neonatal Herpes, the second most common TORCH infection, is caused by perinatal transmission of Herpes Simplex Virus 2, often from maternal cutaneous lesions (14). It leads to extensive cerebral damage, multicystic encephalomalacia, and scattered intracranial calcifications (6).
Congenital toxoplasmosis, caused by transplacental Toxoplasma gondii transmission, has an incidence of 0.1 – 0.6%. Infection rates vary by trimester, with the lowest in the first (20%) and highest in the third (up to 60%) (14). Congenital toxoplasmosis is associated with hydrocephalus and intracranial calcifications (4, 6, 14). Calcifications are nodular in the periventricular area and cortex, and curvilinear in the thalamus and basal ganglia (4; larger calcifications are linked to earlier infections (14). Studies suggest that congenital toxoplasmosis calcifications may decrease or resolve after treatment. Patel et al. found that 75% of treated infants showed decreased or resolved calcifications, while 25% remained stable (15) (Fig. 4b).
Congenital rubella infection has become rare due to rubella vaccination. Transplacental transmission following maternal infection leads to encephalitis, seizures, cataracts, hearing loss, cardiac defects, and intracranial calcifications (14). Calcifications are typically found in the basal ganglia and periventricular area (6, 14).
Congenital Zika infection, identified in 2015, is linked to microcephaly in newborns following maternal Zika virus infection. Zika virus is transmitted by infected arthropods and via placental transfer, causing neurological defects. Aragao et al. found intracranial calcifications as the main CT finding in 23 children with congenital Zika infection. Calcifications were primarily between the cortex and subcortical white matter, mostly punctate, with some linear and coarse calcifications (16).
Human Immunodeficiency Virus (HIV) encephalitis can be congenital or acquired (Fig. 5). Congenital HIV encephalitis results from transplacental transmission, leading to CNS invasion and myelin damage. Neuroimaging may show microcephaly, ventriculomegaly, cerebral atrophy, and symmetrical intracranial calcifications in the basal ganglia and subcortical matter (14).
Figure 5.
NCCT showing intracranial calcifications in an adult with previous HIV encephalitis.
24-year- old male with previous HIV encephalitis.
Technique: Axial non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Coarse calcifications in the cerebral white matter (short arrows) and in the basal ganglia (long arrows).
Acquired
Neurocysticercosis, a parasitic infection endemic in regions like Mexico, South America, Asia, and Africa, is caused by the tapeworm Taenia Solium through ingestion of undercooked meat. Taenia Solium migrates to the CNS, causing inflammation upon larval death, seen as a ring-enhancing lesion with edema (14). Later, characteristic intracranial calcifications develop in the brain parenchyma and subarachnoid space as an eccentric calcified nodule within a peripherally calcified cyst (4) (Fig. 6).
Figure 6.
NCCT of intracranial calcifications in an adult with neurocysticercosis.
33-year-old male with cysticercosis.
Technique: Axial non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Dots of calcifications in subependymal region (long arrow) and at gray to white matter interface (short arrows).
Mycobacterium tuberculosis infection primarily affects the lungs, but 5–10% of cases involve the CNS as tuberculous meningitis or intracranial tuberculoma. Tuberculomas occur in both HIV-positive and immunocompetent individuals in endemic areas (17). Tuberculomas may calcify centrally, creating a pathognomonic target sign with surrounding ring enhancement. Meningeal calcifications are less common (4) (Fig. 7a & 7b).
Figure 7.
NCCT images of intracranial calcification within a tuberculoma in a child.
9-year-old male patient with a tuberculoma.
Technique: Axial enhanced (b) and non-enhanced CT (a), 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Tuberculoma with tiny calcification on both contrast and non-contrast enhanced CT scan. Both arrows show a central calcification within the tuberculoma.
Cryptococcus neoformans infection mainly affects individuals with advanced HIV infection and AIDS. Neuroradiological findings vary, depending on the hematogenous spread along perivascular spaces at the brain base (18). Calcifications are rare and occur late in the disease as punctate calcifications in the brain parenchyma and leptomeninges. Leptomeningeal calcifications may be sequelae of chronic subdural and epidural empyema (4, 18).
Vascular
Intracranial atherosclerosis is a leading cause of stroke globally. Vascular calcifications are present in up to 90% of atherosclerotic lesions and are potential predictors of ischemic strokes (19). They are found in up to 82% of ischemic stroke patients and 52% of non-ischemic stroke patients, with an overall prevalence of 3.5%. Incidence increases with age, peaking after 65 years (3). Calcifications occur in intracranial artery walls, most commonly in the internal carotid (60%), vertebral (20%), middle cerebral (5%), and basilar arteries (5%) (20) (Fig. 8).
Figure 8.
NCCT showing intracranial vascular calcifications due to atherosclerosis.
68-year-old female patient presenting with bilateral internal carotid artery atherosclerosis.
Technique: Axial enhanced and non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Bilateral internal carotid artery atherosclerosis. Arrows show atherosclerotic calcifications.
Vascular calcifications are also seen in cavernous angiomas (Fig. 9), arteriovenous malformations (AVMs), dural arteriovenous fistulas (AVFs), and aneurysms. Cavernous angiomas show calcifications in up to 33% of cases (4, described as scattered dots or stippled in vessel walls or adjacent parenchyma, usually in non-hemorrhagic lesions. Calcifications in venous angiomas and capillary telangiectasias are less common. AVM-associated calcifications occur in 25–30%, often along tortuous veins or the nidus, or as dystrophic calcification due to ischemia distant from the malformation (21). AVFs may show non-specific, bilaterally symmetrical subcortical calcifications, similar to SWS, TS, and Fahr disease, but without other calcification foci (4). Aneurysm-related calcifications are common in thrombosed aneurysms and less so in non-thrombosed aneurysms, especially fusiform types (21, 22).
Figure 9.
NCCT image showing punctate calcification within a cavernous angioma.
70-year-old female patient presenting with cavernous angioma.
Technique: Axial enhanced and non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Punctate calcification found in the cavernous angioma.
In pediatric populations, spontaneous intimal tears or dissections can lead to intracranial aneurysms, which calcify in about 20% of cases. Marginal calcifications form initially, followed by sheet-like calcific plaques. Studies suggest that calcified aneurysms, especially those ≥10 mm, are more stable than uncalcified ones (14).
Mineralization of lenticulostriate arteries has been linked to basal ganglia ischemic stroke in children (23). Lingappa et al. found lenticulostriate artery mineralization in 22 of 23 children (mean age 11 months) with basal ganglia ischemic stroke. Gowda et al. also observed this mineralization in all children (mean age 14 months) with basal ganglia stroke (24). CT scans showed sharply marginated, linear hyperdense lesions emerging vertically through the inferior lentiform nucleus, with attenuation values of 58–90 HU, indicating mineralization. Mineralized arteries ranged from one to five per side, with infarcts located on the side of mineralization (24).
Neoplastic
Brain calcifications are valuable in identifying and evaluating brain neoplasms. Their presence or absence, along with patient age and tumor location, aids in radiological tumor identification and differential diagnosis (4, 6).
Intra-axial
Astrocytomas, a diverse group of intra-axial brain tumors, may calcify in up to 20%, with pilocytic astrocytomas calcifying in up to 25% of cases. Due to their high prevalence, astrocytomas are the most common intra-axial tumors to calcify (4). Pilocytic xanthoastrocytoma, affecting adolescents and young adults (median age 17), is typically superficial, cortically based, and involves leptomeninges. It can appear cystic with a mural nodule or solid mass. Calcifications, seen in up to 40% of cases, are more common in solid forms. Subependymal giant cell astrocytoma (SEGA), associated with tuberous sclerosis (TS), occurs in the first two decades of life, arising from the ventricular wall near the foramen of Monro. SEGAs are well-defined, round, and may have heterogeneous calcifications. While pilocytic xanthoastrocytomas often present with seizures, SEGAs more commonly present with hydrocephalus (14).
Oligodendrogliomas constitute 5–7% of hemispheric tumors in adults but are rare in children (1% of pediatric brain tumors). Differentiating oligodendrogliomas from oligoastrocytomas can be challenging radiologically, often requiring genetic testing. They are commonly located in the frontal and temporal lobes (up to 80%) with minimal mass effect (14). Calcifications and cystic lesions are frequent in oligodendrogliomas. Calcification rates are higher in adults (90%) than children (up to 40%) (4, 14). Calcifications are associated with intratumoral vessels and can extend into surrounding parenchyma, presenting a nodular and clumped pattern (4, 6).
Gangliogliomas are less common than oligodendrogliomas in adults (4 but more frequent in children, representing 1–4% of pediatric brain tumors. They are cortically based, often in the temporal lobe, and can be solid (43%) or solid and cystic (52%) (14). Calcifications occur in up to 41% of cases, typically as a mural calcified nodule (4, 14).
Medulloblastoma, a highly malignant posterior fossa tumor, is the most common posterior fossa tumor in children (38%), with a male predilection. Symptoms include headache, vomiting, and ataxia (14). CT scans show a hyperdense lesion in the cerebellar vermis or hemisphere with tiny scattered dots or clumped calcifications in 22–29% of cases (14, 22) (Fig. 10).
Figure 10.
NCCT image showing calcifications within a medulloblastoma in a child.
8-year-old male patient presenting with medulloblastoma.
Technique: Axial enhanced and non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Scattered and clumped calcifications.
Metastatic lesions rarely calcify, except for metastases from lung and breast cancers, osteogenic sarcoma, and mucinous adenocarcinoma. Calcifications can also develop post-radiation and chemotherapy (4).
Extra-axial
Meningiomas are well-circumscribed extra-axial tumors. Pediatric meningiomas are more common in males and occur in atypical locations compared to adults, with higher recurrence rates, more malignant subtypes, and association with neurofibromatosis (14). Calcification rates range from 20% (14) to 69% (4. Calcification patterns include sand-like, sunburst, rim, and globular types throughout the tumor.
Craniopharyngiomas account for up to 50% of pediatric suprasellar tumors, presenting with visual field disturbances, endocrine abnormalities, and headaches. Adamantinomatous craniopharyngioma, the most common type in children, appears cystic on NCCT with partial calcifications, ranging from thin and circumferential to chunky (14). Pediatric craniopharyngiomas calcify in up to 93% of cases, more frequently than in adults (4, 14) (Fig.11a).
Figure 11.
NCCT examples of intracranial calcifications in extra-axial and intraventricular tumors.
Technique: Axial non enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
(a): 38-year-old female with craniopharyngioma.
Findings: A sellar/suprasellar mass-like (long arrow) and rim calcifications (short arrows) impinging on the foramen of Monro causing hydrocephalus.
(b): 20-year-old male with pineocytoma.
Findings: A mass in the pineal gland (short arrows) with peripheral calcification (long arrow).
(c): 13-year-old boy with pineal teratoma.
Findings: Conglomerate of dense calcifications (long arrow) inside the pineal mass (short arrows).
(d): 27-year-old male with intraventricular ependymoma.
Findings: An irregular mass centered in the body of left lateral ventricle (short arrows) containing dense mass-like calcifications (long arrow) with secondary enlargement of the lateral ventricles.
(e): 42-year-old male with central neurocytoma.
Findings: A mass in the septum pellucidum (short arrows) containing conglomerates of calcifications (long arrow), obstructing the foramen of Monro and causing hydrocephalus of lateral ventricles.
(f): 62-year-old female with intraventricular meningioma.
Findings: Interventricular meningioma centered in posterior body of left lateral ventricle (short arrows) showing internal blush-like calcifications (long arrow) and a rim of calcifications (arrowheads) with secondary enlargement of the left occipital horn.
Pineal tumors range from benign pineocytomas to aggressive pineoblastomas. Benign lesions are more common in adolescents and young adults, while malignant lesions affect children and adolescents. Pineocytomas are well-defined, homogeneous lesions, unlike infiltrative, heterogeneous pineoblastomas. Calcifications are common in pineal tumors (up to 50%), often peripheral, creating an “exploded” pattern (14). Pineal gland calcifications exhibit an exploded pattern, while tumor-induced calcifications are centrally located (4) (Fig.11b).
Germ cell tumors (GCTs) include germinomatous (GGCTs) and non-germinomatous GCTs (NGGCTs). In males, 70% of cranial GGCTs occur in the pineal gland area, while in females, 75% are supratentorial. Teratomas, common NGGCTs, show heterogeneous CT density due to fat, soft tissue, and osseous/cartilaginous components (14). Heterogeneous calcifications with germ cell elements in the pineal region of young patients suggest a GCT, commonly teratoma (4, 14) (Fig.11c).
Schwannomas, pituitary macroadenomas, dermoid, and epidermoid tumors rarely calcify. Lipomas often calcify, displaying a characteristic egg-shell or central calcification pattern (4).
Intraventricular
The most common intraventricular tumors to calcify are ependymomas, choroid plexus tumors, central neurocytomas, meningiomas, and metastases.
Intraventricular ependymomas show dot-like or mass-like/rock-like calcifications (Fig.11d). Posterior fossa ependymomas are most likely to calcify (up to 50%). Choroid plexus papillomas and carcinomas calcify in 25% of cases, typically as dots. Central neurocytomas calcify in 50% of cases, ranging from dots to large masses (25) (Fig.11e). Intraventricular meningiomas show calcification patterns similar to extra-axial meningiomas, occurring in 50% of cases (6) (Fig.11f).
Metabolic/Endocrine
Disorders affecting calcium homeostasis, such as hypoparathyroidism and hyperparathyroidism (Fig.12), and to a lesser extent hypothyroidism (Fig.13), can lead to brain calcifications (14).
Figure 12.
NCCT showing intracranial calcifications in an adult with hyperparathyroidism.
29-year-old male with hyperparathyroidism.
Technique: Axial non enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Conglomerate of calcifications in the bilateral lentiform nuclei (long arrows) and in the bilateral thalami (short arrows).
Figure 13.
NCCT showing intracranial calcifications in an adult with hypothyroidism.
63-year-old male with hypothyroidism.
Technique: Axial non-enhanced CT, 450 mAs, 120 kV, 0.8 mm slice thickness.
Findings: Multiple scattered small linear calcifications in the cerebellar (short arrows) and periventricular white matter (long arrows) bilaterally.
Hypoparathyroidism involves low parathyroid hormone levels or end-organ PTH resistance (14). Its prevalence is estimated at 37 per 100,000, with post-surgical etiology being most common. Radiological features include osteosclerosis, calvarial thickening, soft tissue calcifications, and hypoplastic dentition (26). Brain calcifications in hypoparathyroidism primarily affect the basal ganglia bilaterally, especially the globus pallidus, but can also occur in the dentate nucleus, corona radiata, subcortical white matter, and thalamus (26). While hyperphosphatemia was initially thought to be the cause (26), the exact etiology remains unclear (14). Symmetrical basal ganglia calcifications are also seen in Fahr’s disease, necessitating clinical and biochemical correlation for differential diagnosis (26).
Inflammatory
Systemic lupus erythematosus (SLE), a multifactorial autoimmune disease, results from vasculitis, thrombosis, and neuronal dysfunction. Neurological involvement occurs in up to 75% of SLE patients (27). Raymond et al.’s study of 27 patients with cerebral lupus erythematosus found intracranial calcifications, either isolated or with brain atrophy or infarcts. Calcifications alone or with atrophy occurred in 11.11% of patients, while calcifications with both atrophy and ischemic lesions occurred in 7.4%. Calcifications were symmetrical and bilateral, most commonly in the cerebellum, followed by centrum semiovale, globus pallidus, putamen, caudate head, and thalamus (27).
Cerebral sarcoidosis occurs in 5–10% of sarcoidosis patients, presenting with cranial neuropathy, or rarely, multifocal hypertension (28). Lawrence et al. reported suprasellar calcifications in 1 of 3 neurosarcoidosis cases (29). Autopsy studies have also noted hypothalamic and cerebellar calcifications (30).
Neurotoxicity
Neurotoxicity in children can result from extrinsic toxins like lead and carbon monoxide (31). Reyes et al. observed cerebral and cerebellar calcifications in 30 American adults with chronic lead exposure. Calcifications were punctiform, curvilinear, speck-like, and diffuse, mainly in the subcortical area, basal ganglia, vermis, and cerebellum (32). Finelli et al. described calcifications in the basal ganglia, deep white matter, cerebral cortex, and hippocampi in lead poisoning cases (33).
Conclusion
Intracranial calcifications are frequently detected on NCCT scans and present a broad spectrum of etiologies, from benign physiologic processes to significant pathologies like brain neoplasms. This review highlights that patient age, clinical context, and the location and pattern of intracranial calcifications are crucial elements for accurate radiological differential diagnosis and appropriate clinical management.
TEACHING POINT
A detailed radiological description of intracranial calcifications, including size, location, and shape, is essential for accurate diagnosis, particularly when combined with patient age and clinical presentation. NCCT remains the primary imaging modality for characterizing these calcifications and guiding differential diagnosis in radiology.
ACKNOWLEDGEMENTS
We extend our gratitude to Dr. Mohammad Rawashdeh, Mr. Youssef Annous, and Mr. Amer Traboulsi for their valuable contributions to this review.
ABBREVIATIONS
AGS Aicardi-Goutières syndrome
AIDS Acquired immunodeficiency syndrome
AVF Arterio-venous fistula(s)
AVM Arterio-venous malformation(s)
BGC Basal ganglia calcification(s)
CMV Cytomegalovirus
CNS Central nervous system
CS Cockayne syndrome
CT Computed tomography
GGCT Germinomatous germ cell tumor(s)
HIV Human immunodeficiency virus
IC Intracranial calcification(s)
MRI Magnetic Resonance Imaging
NCCT Non-contrast computed tomography
NF1 Neurofibromatosis type 1
NF2 Neurofibromatosis type 2
NGGCT Non-germinomatous germ cell tumor(s)
PTH Parathyroid hormone
SEGA Subependymal giant cell astrocytoma
SWB Sturge-Weber syndrome
TORCH Toxoplasma, others, rubella, cytomegalovirus and herpes
TS Tuberous sclerosis
REFERENCES
[1] Grodd W, Krüger R, Hattingen E, Petersen D. Cranial computed tomography (CCT) and magnetic resonance imaging (MRI) of intracranial calcifications. Radiologe. 2017 Nov;57(11):911-921. doi: 10.1007/s00117-017-0307-z. Epub 2017 Oct 12. PMID: 29022035.
[2] Savolaine ER, Gerber JE, Coumas J, Haughton VM, Anderson RE. Microscopic intracranial calcification in adults. Radiology. 1978 Dec;129(3):677-81. doi: 10.1148/129.3.677. PMID: 711871.
[3] Yalcin AD, Kilinc E, Sayit E, Gulcan E, Erdem G, Demir MK. Evaluation of intracranial calcifications on computed tomography: a retrospective study of 11941 subjects. J Comput Assist Tomogr. 2010 Jul-Aug;34(4):512-8. doi: 10.1097/RCT.0b013e3181d9052b. PMID: 20647937.
[4] Grech R, Gatt G, Fsadni P, Montefort S, Zrinzo A. Intracranial calcification. X-ray diagnosis and differential diagnosis. X-ray diagnosis and differential diagnosis. Malta Med J. 2007;19(2):30-36.
[5] Whitehead MT, Sharma R, Soares BP, Zimmerman RA. Physiologic intracranial calcifications in children: location and age correlation. AJNR Am J Neuroradiol. 2015 Jan;36(1):189-94. doi: 10.3174/ajnr.A4081. Epub 2014 Sep 18. PMID: 25236225; PMCID: PMC7960194.
[6] Patay Z, Lantos J, Babinski M, Kocsis G. Incidental findings on head CT scans of children and adolescents: clinical significance and recommendations for follow-up. Eur J Radiol. 2009 Aug;71(2):211-8. doi: 10.1016/j.ejrad.2008.04.024. Epub 2008 Jun 13. PMID: 18550320.
[7] Comi AM. Sturge-Weber syndrome. Handb Clin Neurol. 2015;132:157-68. doi: 10.1016/B978-0-444-62702-5.00011-9. PMID: 26567453; PMCID: PMC4756848.
[8] Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen BA, North PE, Marchuk DA, Comi AM, Pevsner J. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013 May 9;368(21):1979-87. doi: 10.1056/NEJMoa1213703. Epub 2013 Apr 28. PMID: 23621272; PMCID: PMC3709993.
[9] Jozwiak S, Kotulska K, Szymanska E, Derkus E, Cukrowska B, Roszkowski M, Winek K, Rysz A, Leszczynski B. Clinical and genotype correlations of epilepsy in tuberous sclerosis complex. Epilepsia. 2011 Jun;52(6):1063-70. doi: 10.1111/j.1528-1167.2011.03064.x. Epub 2011 Apr 21. PMID: 21518305.
[10] Ferner RE, Gutmann DH. Neurofibromatosis type 1 (NF1): diagnosis and management. Handb Clin Neurol. 2013;115:893-915. doi: 10.1016/B978-0-444-52910-7.00047-3. PMID: 23931762.
[11] De Vries MC, de Jong G, Seidlitz J, Sigaudy S, Goizet C, Zampino G, Nizon M, Barth M, Peeters EA, Van den Ende J, Mancini GM, Polonchuk L, Raams A, Renard M, Sarret C, Savasta S, Stefanacci P, Tarani L, Teixeira J, Tenorio V, Vanhomwegen M, Vazquez Ronco MA, Williams M, Wilson L, Yarovaya V, Vermeulen RJ, Weber AM, Kleijer WJ, Jaspers NG, Krawczyk PM, Cohn TMA, van der Burg M, van Berkel CG, Goeman JJ, Naegelen C, Amor DJ, Auriti C, Aylett SE, Bayram AK, Berland S, Biskup S, Bley AE, Blok BS, Bonneau D, Bourgeois M, Brady AF, Brooks S, Budisteanu M, Capra V, Cardoso C, Cau C, Ceulemans B, Chelly J, Chong WK, Claes K, Coubes C, Curry CJ, D’Angelo E, De Meirleir L, De Rademaeker M, Dheedene A, Diebold B, Dimassi S, Docquier O, Douglas G, Ehmke N, Elbracht M, Engelbrecht V, Farkas G, Fassi E, Ferrantini F, Fisher R, Freilinger M, Gillessen-Kaesbach G, Giuliano F, Goebbels R, Grattan-Smith P, Green A, Haeusler M, Hahn G, Hamel BC, Haye D, Herkert JC, Hewitt C, Hietala M, Hinteregger A, Horn D, Huemer M, Hurst JA, Irwin D, Isidor B, Iughetti L, Jamra RA, Janssens S, Jewell R, Kääriäinen H, Karall D, Khan R, Kilic SS, Krajewska-Walasek M, Lalatta F, Landsverk ML, Lannoy N, Laugel V, Leboucq N, Lebrun M, Lesca G, Litvinenko I, Loget P, Lopez-Granados E, Maassen V, Macaya A, Manouvrier-Hanu S, Mari F, Marle N, Mathieu-Dramard M, McCann E, McDonell C, McEntagart M, Mendelsohn NJ, Merienne K, Mignot C, Milleron B, Moog U, Morice-Picard F, Morton J, Muller JS, Mundnich C, Naudion S, Neri G, Newman W, Nizon P, Ockeloen CW, O’Connell S, Orrico A, Örs R, Pasquier L, Patel C, Petit F, Philip N, Pienkowska K, Pigors M, Plaiasu V, Polder E, Pop T, Raas-Rothschild A, Rauch A, Reardon W, Reversade B, Revencu N, Rittinger O, Roberts E, Rossi M, Ruivenkamp C, Santoro L, Santos HG, Sarda P, Schiff M, Schinzel A, Schmitt S, Schnekenberg R, Schoner K, Schwabegger B, Scott C, Selicorni A, Semple RK, Shannon N, Sharif SM, Shaw C, Shelagh M, Simsek E, Singhellakis A, Sitkiewicz E, Skinner SA, Smithson SF, Snoeck I, Spaccini L, Stark Z, Steichen-Gersdorf E, Stellacci E, Stewart H, Stolte-Dijkstra I, Streffleur I, Stromme P, Swinkels M, Szakszon K, Sztriha L, Thauvin-Robinet C, Thevenon J, Tobin V, Touraine R, Trainor B, Traverso M, Trevisson E, Uhlmann R, Valente EM, Van Bon BW, van den Elzen AP, van der Crabben SN, van Essen AJ, van Gassen KL, Van Haeringen A, van Hagen JM, van Rahden BH, van Ruissen F, van Slegtenhorst M, Vanderschaeghe D, Vergano S, Vincent-Delorme C, Vissers LE, Vreeburg M, Walker S, Waters AM, Watson C, Weegerink NJ, Wieczorek D, Wildhardt G, Wilson M, Winchester L, Wright M, Zenker M, Ziegler A, Zillgitt A, Zweier C, De Brouwer APM, Kayserili H, Hennekam RC. Cockayne syndrome: clinical spectrum, genotype-phenotype correlation and diagnostic criteria. Eur J Hum Genet. 2015 Feb;23(2):145-54. doi: 10.1038/ejhg.2014.85. Epub 2014 May 21. PMID: 24846739; PMCID: PMC4661489.
[12] Wenger DA, Rafi MA, Luzi P, Datta A, Ginns EI, Millington DS, Kohler R, Lorey F, Hoffmann G, Krügel U, Suzuki K. Krabbe disease (globoid cell leukodystrophy): fifteen mutations in the gene for galactosylceramidase (GALC). Hum Mutat. 1993;2(3):189-99. doi: 10.1002/humu.1380020306. PMID: 8346378.
[13] Saleem S, Aslam HM, Anwar M, Anwar S, Saeedullah, Sheikh Z, Mazhar K. Fahr’s disease: literature review of clinical features, diagnosis, and management. Case Rep Neurol. 2013 May 10;5(1):21-9. doi: 10.1159/000350350. PMID: 23807758; PMCID: PMC3693789.
[14] Dinizio JA, Shetty T, Castillo M. Intracranial calcifications in children: a pictorial review. J Neuroimaging. 2014 May-Jun;24(3):209-24. doi: 10.1111/jon.12085. Epub 2013 Jul 11. PMID: 23841618.
[15] Patel K, Barton LL, Mussi-Pinhata MM, Roizen N, Cypel T, Park AH, Boyer K, Kannassart B, Holfels E, Mets MB, Withers S, Remington JS, McLeod R; National Collaborative Chicago-Based Congenital Toxoplasmosis Study. Resolution of intracranial calcifications in infants with congenital toxoplasmosis treated with anti-parasitic drugs. Pediatr Infect Dis J. 2008 Jul;27(7):623-7. doi: 10.1097/INF.0b013e31816b9e28. PMID: 18545127; PMCID: PMC2748015.
[16] Aragao FV, van der Linden V, Brasil P, Fonsesca EB, Araujo Júnior E, Sá RA, Adam R, Caldas JP, защихина К, Aburjaile F, Moore CA, Gill LH, Hrabalek M, Sequeira WL, Ribeiro EM, Ventura CV, Dias JL, Pessoa AL, Dobyns WB, Barkovich AJ. Intracranial calcifications and associated findings in congenital Zika virus infection. Radiology. 2016 Oct;281(2):337-43. doi: 10.1148/radiol.2016160393. Epub 2016 Jul 12. PMID: 27400398; PMCID: PMC5082431.
[17] Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev. 2008 Oct;21(4):243-81, table of contents. doi: 10.1128/CMR.00042-07. PMID: 18854485; PMCID: PMC2565914.
[18] Rolfes L, Klafke R, Weber F, Janisch W, Hastka J. Cerebral cryptococcosis in AIDS: CT and MRI findings. J Neuroimaging. 2000 Jan;10(1):9-12. doi: 10.1177/105122840001000103. PMID: 10634249.
[19] Kolodgie FD, Burke AP, Farb A, Weber DK, Jang IK, Ardelt MK, Lee JM, Alviar K, Slack JD, Virmani R. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. 2001 Sep;16(5):285-92. doi: 10.1097/00001573-200109000-00002. PMID: 11528385.
[20] Wong KS, Gao S, Chan YH, King A, Ahuja A, Chan FL, Leung TW, Li L, Lui SL, Kay R, Woo J, Lam WW, Leung SY, Poon WS, Tomlinson B, Thomas GN, Wong LK. Intracranial atherosclerosis in ischemic stroke patients with different ethnic origins. Stroke. 2009 Aug;40(8):2788-94. doi: 10.1161/STROKEAHA.109.553444. Epub 2009 Jun 11. PMID: 19520971.
[21] Gross BA, Du R. Natural history of intracranial cavernous malformations. Neurosurg Focus. 2016 Jun;40(6):E3. doi: 10.3171/2016.3.FOCUS1659. PMID: 27256254.
[22] Osborn AG, Salzman KL, Thordarson BB, Provenzale JM. Diagnostic Imaging: Brain. 3rd ed. Salt Lake City, UT: Amirsys; 2016.
[23] Lingappa L, Prendergast J, Holmes GL, Rivkin MJ. Mineralizing angiopathy associated with basal ganglia stroke in young children. Pediatr Neurol. 2005 Dec;33(6):426-31. doi: 10.1016/j.pediatrneurol.2005.05.012. PMID: 16338661.
[24] Gowda VK, Bhat V, Srinivasarangan M, Benakappa N, Adhikari P, Udayakumar AM, Rao S. Mineralizing vasculopathy in term neonates with basal ganglia stroke. J Child Neurol. 2011 Nov;26(11):1423-8. doi: 10.1177/0883073811403721. Epub 2011 May 2. PMID: 21531743.
[25] Yasargil MG, von Ammon K, von Deimling A, Valavanis A, Yasargil DC. Central neurocytoma: histopathological variants and therapeutic approaches. J Neurooncol. 1992;13(1):1-11. doi: 10.1007/BF00165808. PMID: 1398174.
[26] Goswami R, Sharma R, Sreenivas V, Gupta N, Raghunandan C, Ray R, Aggarwal S, Saran RK, Kochupillai N. Prevalence and progression of intracranial calcifications and its correlation with degree of biochemical control in patients with idiopathic hypoparathyroidism. Clin Endocrinol (Oxf). 2009 Jul;71(1):90-6. doi: 10.1111/j.1365-2265.2008.03477.x. Epub 2008 Nov 28. PMID: 19049545.
[27] Raymond AA, Zinkernagel AS, Carré D, Hottinger A, Kuntzer T. Cerebral lupus: neuroimaging and clinical correlation in 27 patients. J Neurol. 2007 Oct;254(10):1351-60. doi: 10.1007/s00415-006-0477-2. Epub 2007 Feb 28. PMID: 17334512.
[28] Stern BJ, Royal W 3rd, Gelfand JM, Lehmann TJ, Chen JC, Dombrowski EC, Frohman EM, Krieger SC, Kumar N, Nath A, Preston DC, Remler B, Saidha S, Scott TF, Sejdić E, Spaeth K, Tyor WR, Valdés-Sueiras M, Wang K, Wolinsky JS, Zubizarreta J, Zivadinov R; Neurology Neuroimmunology Section of the American Academy of Neurology. Definition and consensus criteria for neurologic sarcoidosis: From the Neurology Neuroimmunology Section of the American Academy of Neurology. JAMA Neurol. 2018 Dec 1;75(12):1507-1515. doi: 10.1001/jamaneurol.2018.2587. PMID: 30326595; PMCID: PMC6440259.
[29] Lawrence T, Katzman M, MacLean C, Chan J, Bilbao J. Hypothalamic-pituitary sarcoidosis: case reports and review of the literature. Medicine (Baltimore). 2007 May;86(3):153-65. doi: 10.1097/MD.0b013e3180595b4c. PMID: 17505245.
[30] Herring AB, Urich H. Sarcoidosis of the central nervous system. J Neurol Sci. 1969 Jan;9(1):77-129. doi: 10.1016/0022-510X(69)90147-0. PMID: 5765075.
[31] Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain. 2003 Jul;126(Pt 7):1175-9. doi: 10.1093/brain/awg161. PMID: 12816822.
[32] Reyes PA, Talpeanu B, Vasquez R, Schwartzbard AZ, Patel S, Wilentz RE, Cohen M, Fuster V, Narula J. Intracranial calcification in chronic lead intoxication: CT findings. AJNR Am J Neuroradiol. 2006 Nov-Dec;27(10):2181-3. PMID: 17105878; PMCID: PMC7976663.
[33] Finelli PF, Cardi JK, Falsetti HL, Gaynor JJ. Adult lead neuropathy. Arch Neurol. 1980 Feb;37(2):105-15. doi: 10.1001/archneur.1980.00500510041009. PMID: 7354812.