I. Understanding Metabolic Syndrome: Definitions and Significance
Metabolic syndrome is a cluster of interconnected risk factors that significantly elevate the likelihood of developing atherosclerotic cardiovascular disease (CVD) and type 2 diabetes (T2D). These risk factors include insulin resistance, obesity, atherogenic dyslipidemia, and hypertension, all interwoven through shared biological pathways and mechanisms. While the precise definition and clinical utility of metabolic syndrome have been debated, recognizing this syndrome remains crucial. It allows healthcare professionals to identify a specific patient subgroup with a common underlying pathophysiology and a heightened risk profile for serious health conditions. By understanding the core components of metabolic syndrome and their interrelations, we can gain deeper insights into the disease’s pathogenesis and explore more effective treatment and management strategies.
For decades, the medical community has recognized individual risk factors for CVD, such as family history, hypertension, hyperlipidemia, diabetes, and smoking, alongside non-modifiable factors like age and gender. Addressing these individual risks through lifestyle changes and medication has been the cornerstone of CVD prevention. However, it’s increasingly evident that these risks often cluster together within individuals, suggesting shared root causes and responses to interventions like diet and exercise. This clustering phenomenon points towards a common underlying pathophysiology, making the concept of metabolic syndrome a valuable tool for understanding and managing complex patient risks.
Metabolic syndrome is characterized by the co-occurrence of hyperglycemia/insulin resistance, obesity, and dyslipidemia. Its importance lies in several key areas:
- Risk Identification: It effectively identifies individuals at increased risk of developing both atherosclerotic CVD and T2D.
- Pathophysiological Understanding: By examining the relationships between metabolic syndrome components, we can better elucidate the underlying mechanisms linking these conditions and their contribution to CVD risk.
- Research Advancement: The concept facilitates epidemiological and clinical research into pharmacological, lifestyle, and preventive approaches for this complex syndrome.
II. Current Diagnostic Definitions of Metabolic Syndrome
Several organizations have proposed definitions for metabolic syndrome, each with nuances in their criteria. The most widely recognized definitions are summarized below, highlighting their evolution and key differences.
1. World Health Organization (WHO) Definition (1998)
The WHO definition, the first to formally define metabolic syndrome in 1998, emphasizes insulin resistance as a mandatory criterion. Diagnosis requires evidence of insulin resistance plus at least two additional risk factors from a list including obesity, dyslipidemia, hypertension, and microalbuminuria.
Criteria:
- Required: Insulin resistance, indicated by one of the following:
- Impaired Fasting Glucose (IFG): Fasting glucose ≥ 100 mg/dL
- Impaired Glucose Tolerance (IGT): 2-hour glucose in Oral Glucose Tolerance Test (OGTT) ≥ 140 mg/dL
- Elevated Homeostatic Model Assessment of Insulin Resistance (HOMA-IR)
- Euglycemic hyperinsulinemic clamp (research setting)
- Plus any two of the following:
- Obesity
- Dyslipidemia
- Hypertension
- Microalbuminuria
Strengths: Highlights the central role of insulin resistance in the pathophysiology of metabolic syndrome.
Limitations: Complex to apply clinically due to the requirement for direct insulin resistance measurement. Not ideal for large-scale epidemiological studies requiring simpler assessments.
2. European Group for the Study of Insulin Resistance (EGIR) Definition (1999)
EGIR, in 1999, also prioritized insulin resistance, defining it as fasting plasma insulin above the 75th percentile. Similar to WHO, the EGIR definition requires insulin resistance plus two additional criteria from obesity, hypertension, and dyslipidemia. Notably, the EGIR definition excludes patients with T2D from being diagnosed with metabolic syndrome and simplifies obesity criteria to waist circumference.
Criteria:
- Required: Insulin resistance, indicated by fasting plasma insulin ≥ 75th percentile
- Plus any two of the following:
- Obesity (waist circumference)
- Hypertension
- Dyslipidemia
Strengths: Simplifies the assessment of insulin resistance using fasting insulin.
Limitations: Excludes patients with T2D. Fasting insulin may not accurately reflect insulin resistance in diabetic individuals.
3. National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) Definition (2001, updated 2005)
The NCEP ATP III definition, updated in 2005 by the American Heart Association and the National Heart Lung and Blood Institute, is one of the most widely used clinical definitions. It requires meeting at least three out of five criteria, focusing on readily measurable clinical parameters. This definition does not mandate insulin resistance or obesity as prerequisites.
Criteria (at least 3 out of 5):
- Waist circumference: ≥ 40 inches (men) or ≥ 35 inches (women)
- Blood pressure: ≥ 130/85 mmHg
- Fasting triglycerides (TG): ≥ 150 mg/dL
- Fasting high-density lipoprotein (HDL) cholesterol: < 40 mg/dL (men) or < 50 mg/dL (women)
- Fasting blood sugar: ≥ 100 mg/dL
Strengths: Clinically practical, utilizing easily obtainable measurements. Simple to remember and apply in both clinical and epidemiological settings. Does not presuppose underlying causes, accommodating various pathophysiological viewpoints.
Limitations: Does not explicitly require insulin resistance or obesity, potentially missing the underlying pathophysiology in some individuals.
4. International Diabetes Federation (IDF) Definition (2005)
The IDF definition, introduced in 2005, mandates central obesity as a prerequisite, alongside two or more additional criteria. It utilizes population-specific waist circumference cutoffs to account for ethnic and regional variations in body composition and associated risks. While including similar criteria, the IDF definition emphasizes obesity over insulin resistance in the syndrome’s pathophysiology.
Criteria:
- Required: Central obesity (population and country-specific waist circumference thresholds)
- Plus any two of the following:
- Fasting triglycerides (TG): ≥ 150 mg/dL
- Fasting HDL cholesterol: < 40 mg/dL (men) or < 50 mg/dL (women)
- Blood pressure: Systolic ≥ 130 mmHg or Diastolic ≥ 85 mmHg, or treatment of hypertension
- Fasting plasma glucose: ≥ 100 mg/dL, or previously diagnosed T2D
Strengths: Recognizes the importance of central obesity and accounts for population-specific variations.
Limitations: Emphasis on obesity over insulin resistance is debated. Criticized by some for downplaying the role of insulin resistance in the core pathophysiology.
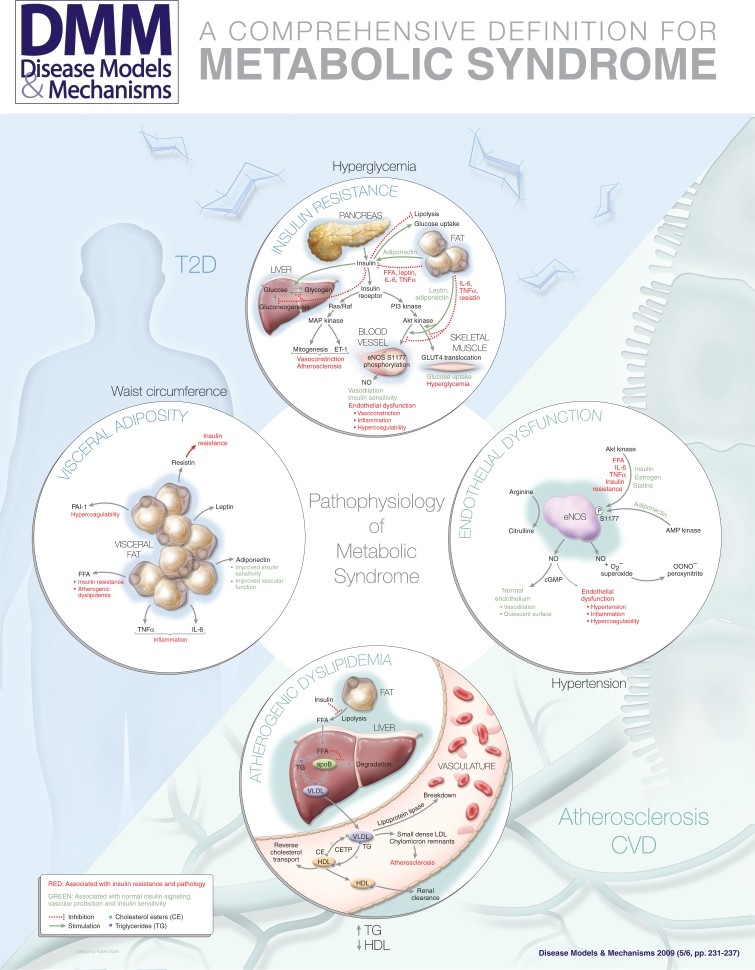 graphic file with name DMM001180.jpg
graphic file with name DMM001180.jpg
Image: Diagram illustrating the interconnectedness of central features in metabolic syndrome, including insulin resistance, visceral adiposity, atherogenic dyslipidemia, and endothelial dysfunction.
III. Clinical Utility of Metabolic Syndrome Diagnosis
1. Identifying Patients at Elevated Risk
A primary application of metabolic syndrome diagnosis is to identify individuals at increased risk of developing CVD and T2D in routine clinical practice. However, it’s important to acknowledge that metabolic syndrome assessment is not a standalone risk prediction tool. Existing risk assessment methods incorporate other crucial factors not included in metabolic syndrome definitions, such as family history of diabetes, age, gender, smoking status, and LDL cholesterol levels. Therefore, metabolic syndrome diagnosis should be considered complementary to, rather than a replacement for, comprehensive risk assessments like the Framingham risk score for CVD or diabetes prediction models.
The true value of metabolic syndrome lies in its ability to pinpoint a specific subgroup of patients who share a common underlying pathophysiology. It serves as a clinical shorthand for a cluster of interconnected biological processes that significantly increase health risks.
The NCEP ATP III definition, in particular, is easily integrated into clinical workflows. Healthcare providers can quickly assess patients against the five criteria using readily available clinical measurements, providing a straightforward “yes” or “no” determination of metabolic syndrome presence. This simplicity contrasts with more complex risk calculation tools. It is hoped that diagnosing metabolic syndrome will motivate both patients and clinicians to proactively implement lifestyle modifications (diet, exercise) and appropriate pharmacological interventions to mitigate the associated risks of CVD and T2D.
2. Enhancing Understanding of Pathophysiological Mechanisms
Metabolic syndrome provides a framework for understanding the interconnectedness of insulin resistance, visceral adiposity, dyslipidemia, and hypertension. By grouping these conditions, the concept facilitates research into shared pathophysiological pathways, the development of relevant animal models, and the testing of novel therapeutic strategies.
Despite its ICD-9 diagnostic code (277.7), debates continue about whether metabolic syndrome is a distinct disease entity. However, recognizing metabolic syndrome as a syndrome highlights that individuals with this cluster of risk factors face a significantly higher risk of T2D and CVD compared to those with isolated risk factors. For instance, individuals with isolated hypertension or hyperlipidemia have increased CVD risk, but this risk is lower than in those meeting criteria for metabolic syndrome. Even though diabetes is considered a CVD risk equivalent, the presence of additional metabolic syndrome components further elevates CVD risk in these patients.
While concerns exist about potential misclassification of patients with varying combinations of features using different definitions, the structure of most definitions effectively captures individuals with the core underlying pathophysiological processes of metabolic syndrome.
3. Facilitating Epidemiological Research
Numerous epidemiological studies have investigated metabolic syndrome prevalence across diverse populations and its association with T2D, CVD, and other related conditions like non-alcoholic fatty liver disease, gallstones, polycystic ovary syndrome, sleep apnea, and gout. These studies rely on simple, easily applicable definitions for large-scale assessments. Epidemiological research on metabolic syndrome contributes to our understanding of the condition’s pathophysiology, genetic underpinnings (through genome-wide association studies), and the development of treatment approaches targeting the syndrome’s composite physiological abnormalities rather than individual components.
IV. Core Components of Metabolic Syndrome
Drawing upon Einstein’s principle of simplicity without oversimplification, the various definitions of metabolic syndrome converge on four core features: insulin resistance, visceral obesity, atherogenic dyslipidemia, and endothelial dysfunction. Insulin resistance and visceral obesity appear to be fundamental to the syndrome’s manifestation. Weight loss in affected individuals often improves multiple features simultaneously, suggesting a necessary role for adiposity in the syndrome’s pathophysiology. Conversely, some obese individuals do not exhibit other metabolic syndrome components, indicating that both a metabolic predisposition to insulin resistance and obesity are likely required for the full syndrome phenotype. Atherogenic dyslipidemia and endothelial dysfunction are considered downstream consequences of insulin resistance and visceral obesity, contributing mechanistically to atherosclerosis and CVD development.
These four central features—insulin resistance, visceral adiposity, atherogenic dyslipidemia, and endothelial dysfunction—can be viewed as the most concise and comprehensive definition of metabolic syndrome. While other factors like systemic inflammation, hypercoagulability, or microalbuminuria may be relevant to the pathophysiology, they are not essential for the core definition as they are not independently required for diagnosis.
1. Insulin Resistance
Insulin, produced by the pancreas in response to elevated blood glucose, regulates glucose utilization in various tissues, primarily skeletal muscle, liver, and adipose tissue. In these tissues, insulin promotes glucose uptake, glycogen synthesis, and inhibits glucose production by the liver (gluconeogenesis). Insulin resistance occurs when these tissues become less responsive to insulin’s effects, leading to elevated blood glucose levels and a cascade of metabolic disturbances.
Insulin sensitivity varies widely across individuals, influenced by factors like adiposity, fitness, and genetic predisposition. Insulin resistance is a potent predictor of T2D, and hyperinsulinemia often serves as a surrogate marker.
At a cellular level, insulin signaling involves binding to the insulin receptor, activating tyrosine kinase pathways. Two major pathways are activated:
- Phosphoinositide 3-kinase (PI3K) pathway: Mediates metabolic effects of insulin, including glucose uptake and endothelial nitric oxide synthase (eNOS) activation.
- Mitogen-activated protein (MAP) kinase pathway: Influences vasoconstriction, vascular cell adhesion, and smooth muscle growth.
In insulin resistance, the PI3K-Akt pathway is impaired, while the MAP kinase pathway remains relatively unaffected. This imbalance contributes to:
- Reduced endothelial nitric oxide (NO) production: Leading to endothelial dysfunction.
- Decreased GLUT4 translocation: Impairing glucose uptake in muscle and fat tissue.
- Continued MAP kinase pathway activity: Promoting vasoconstriction, inflammation, and vascular smooth muscle proliferation.
Thus, insulin resistance disrupts vascular function, predisposing to atherosclerosis. Insulin also normally enhances blood flow to tissues via eNOS activation, contributing to glucose delivery and metabolism. Mechanisms contributing to insulin resistance, such as hyperglycemia, advanced glycation end products, free fatty acids (FFAs), obesity, dyslipidemia, and inflammation, can also impair vascular function.
2. Visceral Adiposity
Visceral obesity, characterized by excess fat accumulation in the abdominal cavity, is strongly linked to insulin resistance. Adipokines, signaling molecules produced by adipose tissue, play a key role in mediating the interplay between metabolism and vascular function. Pro-inflammatory adipokines like tumor necrosis factor α (TNFα) and interleukin-6 (IL-6) contribute to both insulin resistance and vascular dysfunction. The renin-angiotensin system is also activated in visceral adipose tissue, potentially contributing to hypertension and insulin resistance.
In contrast, adiponectin, a protective adipokine, enhances insulin sensitivity and energy metabolism. Adiponectin levels are reduced in obesity, T2D, and metabolic syndrome. Furthermore, FFAs released from visceral fat and bioactive lipid intermediates impair the PI3K-Akt pathway and increase oxidative stress, further exacerbating insulin resistance and endothelial dysfunction.
3. Atherogenic Dyslipidemia
Atherogenic dyslipidemia, a hallmark of metabolic syndrome, is characterized by:
- Elevated plasma triglyceride (TG) levels
- Low high-density lipoprotein (HDL) cholesterol levels
- Increased small dense low-density lipoprotein (LDL) particles
Insulin resistance and visceral obesity are central to the development of atherogenic dyslipidemia. Insulin normally suppresses lipolysis in adipocytes; in insulin resistance, impaired insulin signaling leads to increased lipolysis and elevated FFA levels. In the liver, FFAs promote TG synthesis and very-low-density lipoprotein (VLDL) production. Insulin resistance also reduces VLDL clearance and alters lipoprotein metabolism, leading to the characteristic lipid profile of atherogenic dyslipidemia. These lipid abnormalities contribute to atheroma formation.
4. Endothelial Dysfunction
Endothelial dysfunction represents a final common pathway linking numerous cardiovascular risk factors to atherosclerosis. The endothelium, the inner lining of blood vessels, plays critical roles in vascular tone, inflammation, hemostasis, and vascular smooth muscle modulation. Normal endothelial function is protective against atherosclerosis, while endothelial dysfunction is central to atherosclerotic lesion development.
Endothelial dysfunction occurs when the endothelium’s normal protective mechanisms are impaired, often due to damage or exposure to factors like oxidative stress, hyperglycemia, advanced glycation end products, FFAs, inflammatory cytokines, or adipokines. A key feature is reduced nitric oxide (NO) bioavailability in the vasculature.
Mechanisms of endothelial dysfunction include:
- Reduced eNOS phosphorylation at S1177: Impairs eNOS activity and NO production.
- Rapid reaction of NO with superoxide: Forming peroxynitrite, a damaging molecule.
- Increased asymmetric dimethylarginine (ADMA): Competes with arginine, reducing NO synthesis.
- eNOS cofactor deficiency (BH4): Leads to “uncoupled” eNOS, generating superoxide instead of NO.
Insulin resistance, visceral adiposity, and associated factors contribute to endothelial dysfunction through various mechanisms, including reduced Akt kinase activity, increased oxidative stress, and altered adipokine profiles, ultimately accelerating atherosclerosis.
V. Conclusion
Metabolic syndrome is defined by the clustering of insulin resistance, visceral adiposity, atherogenic dyslipidemia, and endothelial dysfunction. These interconnected conditions share common biological pathways and mechanisms. A comprehensive yet simple definition of metabolic syndrome should encompass these core features. Utilizing multiple criteria helps distinguish individuals with the full syndrome from those with isolated risk factors. Including both TG and HDL criteria enhances specificity for atherogenic dyslipidemia, and incorporating blood pressure ensures the presence of endothelial dysfunction.
Among the various definitions, the NCEP ATP III definition stands out for its clinical and epidemiological practicality due to its straightforward and readily measurable criteria. Despite ongoing debates about the syndrome’s conceptual utility, metabolic syndrome clearly delineates specific pathophysiological mechanisms linking its central features. Recognizing metabolic syndrome as a distinct entity facilitates research into its genetic basis, underlying pathophysiology, and the development of targeted treatment strategies.
References
Original article references would be listed here, maintaining original citations as appropriate for an SEO-optimized, English-language version focusing on providing accurate information rather than strict academic citation replication.