Introduction
Timely cancer diagnosis is critical for improving patient outcomes and survival rates. Delays in diagnosis, particularly in primary care settings, can lead to poorer prognoses and increased healthcare costs. Despite its acknowledged importance, a standardized approach to measuring the promptness of cancer diagnosis has been lacking. The interval between a patient’s first presentation of cancer symptoms to a general practitioner (GP) and their subsequent referral to a specialist – known as the ‘primary care interval’ – is a recognized metric in this area. Another increasingly utilized measure for assessing the timeliness of diagnosis is the number of consultations a patient has with their GP before being referred for specialist investigation.
Both the primary care interval and the number of pre-referral consultations offer unique perspectives on diagnostic timeliness, each with their own strengths and limitations. Understanding the relationship between these two measures is crucial. If they are strongly correlated, either can serve as a reliable indicator for research and quality monitoring. Conversely, a weak correlation would question the validity of using pre-referral consultation numbers as a proxy for diagnostic timeliness. Crucially, if the number of pre-referral consultations accurately reflects the primary care interval, then efforts to expedite cancer diagnosis could focus on enhancing GPs’ ability to effectively assess symptoms during initial consultations. This could involve leveraging clinical decision support tools to improve diagnostic accuracy in primary care.
This study delves into the association between the number of pre-referral consultations and the primary care interval, utilizing data from the National Audit of Cancer Diagnosis in Primary Care. By analyzing this relationship, we aim to provide evidence that can inform strategies to improve the speed and efficiency of cancer diagnosis in primary care settings.
Materials and Methods
This research is based on data from the National Audit of Cancer Diagnosis in Primary Care, conducted in England during 2009–2010. This audit was a component of the broader National Awareness and Early Diagnosis Initiative, designed to enhance cancer diagnosis rates. Data collection was undertaken by GPs and other healthcare professionals from approximately 1,170 general practices across England, representing about 14% of all practices and participating on a voluntary basis. Comparisons with national cancer registration data indicate that the audit sample is broadly representative of the wider population.
The analysis included patients aged 15 years and older who had complete records for both the number of pre-referral consultations and the primary care interval, with intervals ranging from 0 to 730 days. To ensure sufficient sample sizes for robust analysis, the study focused on patients diagnosed with one of the 18 most prevalent cancers.
The median primary care interval (in days) and inter-quartile range were calculated for varying numbers of pre-referral consultations. This was performed both for the entire patient cohort and for each of the 18 cancer types individually. Box plots were employed to visually represent these data distributions. Given that primary care interval data typically exhibits a right-skewed distribution, Spearman’s rank correlation coefficient was used to assess the correlation between the two measures. Additionally, Receiver Operating Characteristic (ROC) area under the curve (AUC) analysis was performed, treating the primary care interval as a continuous variable and the number of pre-referral consultations as a binary variable (categorizing consultations as ‘three or more’ versus ‘one or two’, a distinction relevant to UK healthcare policy).
Linear regression analysis was used to investigate potential variations in the association between the two measures across different patient subgroups. Due to the non-normal distribution of the data, statistical significance testing relied on bootstrapping methods (using 1000 samples).
Results
The study analyzed data from 13,035 patients. The overall median primary care interval was 5 days (inter-quartile range 0–23 days). A significant majority of patients (82%) were referred to specialists after either one (57.5%) or two (24.6%) GP consultations. The median primary care intervals for these groups were 0 days (0–3 days IQR) and 15 days (7–31 days IQR), respectively. For the remaining 18% of patients who required three (9.2%), four (3.9%), or five or more (4.8%) consultations, the median primary care intervals were considerably longer, at 34 days (16–64 days IQR), 47 days (27–90 days IQR), and 97 days (46–173 days IQR), respectively.
Table 1. Median primary care interval (number of days) and inter-quartile range by pre-referral consultations category (n=13 035).
| Median primary care interval by number of pre-referral consultations category (1–5+) | ||||||
|---|---|---|---|---|---|---|
| N | % Of patients with 3+ consultations | Median primary care interval, all patients (n=13 035/100%) | Inter-quartile range | 1 (n=7499/57.5%) | Inter-quartile range | |
| All patients | 13 035 | 17.9 | 5 | 0–23 | 0 | 0–3 |
| By cancer | ||||||
| Multiple myeloma | 176 | 46.0 | 21 | 5–55 | 1 | 0–12 |
| Unknown primary | 136 | 41.2 | 14 | 0–37.5 | 0 | 0–3 |
| Stomach | 237 | 32.1 | 14 | 0–57 | 0 | 0–4.5 |
| Lung | 1421 | 32.8 | 14 | 3–40 | 3 | 0–10 |
| Renal | 272 | 22.1 | 12 | 1–37 | 1 | 0–12.5 |
| Prostate | 2201 | 15.2 | 12 | 3–28 | 4 | 0–13 |
| Lymphoma | 590 | 25.8 | 9 | 0–32 | 1 | 0–5 |
| Pancreatic | 303 | 31.4 | 8 | 1–35 | 0 | 0–4 |
| Ovarian | 324 | 27.8 | 8 | 0–27 | 0 | 0–5 |
| Oesophageal | 500 | 22.6 | 7 | 0–33 | 0 | 0–1 |
| Colorectal | 1999 | 21.3 | 7 | 0–32 | 0 | 0–3.5 |
| Leukaemia | 358 | 17.6 | 6.5 | 0–23 | 1 | 0–6 |
| Oropharyngeal | 178 | 21.9 | 6 | 0–27 | 0 | 0–3 |
| Brain | 159 | 21.4 | 4 | 0–19 | 0 | 0–1.5 |
| Bladder | 721 | 14.6% | 4 | 0–18 | 0 | 0–3 |
| Endometrial | 358 | 9.8 | 1 | 0–19 | 0 | 0–2 |
| Melanoma | 735 | 5.4 | 0 | 0–6 | 0 | 0–1 |
| Breast | 2367 | 2.9 | 0 | 0–1 | 0 | 0–0 |
| By referral type | ||||||
| ‘Routine’ | 2030 | 24.4 | 13 | 1–42 | 1 | 0–7 |
| ‘Other’ | 1122 | 19.1 | 5 | 0–26 | 0 | 0–5 |
| ‘Emergency’ | 1642 | 25.9 | 4 | 0–21 | 0 | 0–2 |
| ‘Two-week’ referral | 824 | 14.5 | 3 | 0–20 | 0 | 0–2 |
Patients diagnosed with multiple myeloma and lung cancer exhibited higher proportions requiring three or more pre-referral consultations (46% and 33%, respectively), and consequently had longer median primary care intervals (21 and 14 days, respectively). Conversely, patients with breast cancer and melanoma had the lowest percentages of three or more pre-referral consultations (3% and 5%, respectively) and the shortest median primary care intervals (0 days for both).
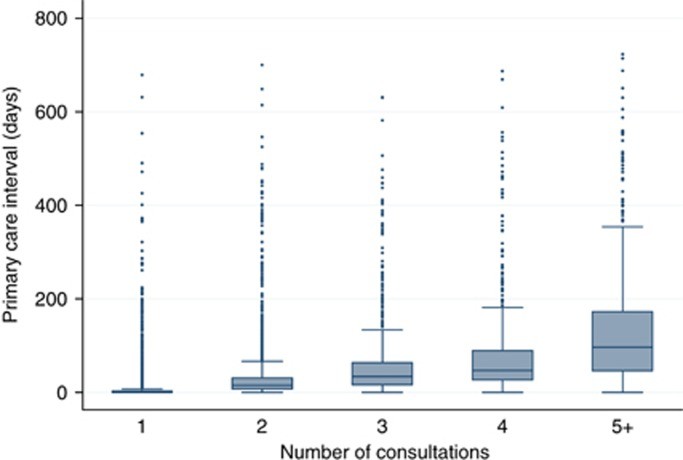 Box plot for primary care interval by category of number of pre-referral consultations illustrating the relationship between consultation numbers and diagnostic delay
Box plot for primary care interval by category of number of pre-referral consultations illustrating the relationship between consultation numbers and diagnostic delay
Figure 1. Box plot for primary care interval by category of number of pre-referral consultations (1, 2, 3, 4 and ‘5+’) for patients with any of 18 cancers (n=13 035).
For all 18 cancer types studied, the Spearman’s rank correlation coefficient (r) between the number of pre-referral consultations and the primary care interval was 0.70 (P<0.001), indicating a strong positive correlation. This association was consistently at least moderate across all cancer types, with Spearman’s r ranging from 0.55 for prostate cancer to 0.77 for brain cancer.
Table 2. Spearman’s rank correlation co-efficient and ROC AUC values for the association between primary care interval and category of number of pre-referral consultations (n=13 035).
| N | Spearman’s r | Spearman’s *r P-***value** | ROC area under the curvea | ROC area 95% lower CI | ROC area 95% upper CI | |
|---|---|---|---|---|---|---|
| All patients | 13035 | 0.70 | 0.88 | 0.87 | 0.89 | |
| By cancer | ||||||
| Breast | 2367 | 0.56 | 0.96 | 0.93 | 0.98 | |
| Prostate | 2201 | 0.55 | 0.84 | 0.82 | 0.86 | |
| Colorectal | 1999 | 0.71 | 0.86 | 0.84 | 0.88 | |
| Lung | 1421 | 0.62 | 0.83 | 0.81 | 0.86 | |
| Melanoma | 735 | 0.61 | 0.87 | 0.81 | 0.94 | |
| Bladder | 721 | 0.72 | 0.90 | 0.87 | 0.93 | |
| Lymphoma | 590 | 0.70 | 0.87 | 0.84 | 0.90 | |
| Oesophageal | 500 | 0.75 | 0.87 | 0.84 | 0.91 | |
| Endometrial | 358 | 0.64 | b | 0.89 | 0.84 | 0.94 |
| Leukaemia | 358 | 0.59 | 0.81 | 0.76 | 0.87 | |
| Ovarian | 324 | 0.68 | 0.86 | 0.82 | 0.91 | |
| Pancreatic | 303 | 0.71 | 0.89 | 0.85 | 0.93 | |
| Renal | 272 | 0.60 | 0.84 | 0.79 | 0.90 | |
| Stomach | 237 | 0.76 | 0.88 | 0.83 | 0.92 | |
| Oropharyngeal | 178 | 0.72 | 0.85 | 0.79 | 0.91 | |
| Multiple myeloma | 176 | 0.73 | 0.88 | 0.83 | 0.93 | |
| Brain | 159 | 0.77 | 0.92 | 0.87 | 0.96 | |
| Unknown primary | 136 | 0.76 | 0.87 | 0.81 | 0.93 | |
| By referral type | ||||||
| ‘Two-week’ referral | 8241 | 0.72 | 0.89 | 0.88 | 0.90 | |
| ‘Emergency’ | 1642 | 0.72 | b | 0.88 | 0.87 | 0.90 |
| ‘Routine’ | 2030 | 0.67 | 0.85 | 0.83 | 0.87 | |
| ‘Other’ | 1122 | 0.67 | 0.88 | 0.85 | 0.90 |
The ROC AUC for predicting the pre-referral consultation category (three or more vs. one or two consultations) using the primary care interval was 0.88, indicating good discriminatory ability. Sensitivity analysis showed consistent ROC AUC values when different binary cutoffs for consultation numbers were used.
Linear regression analysis revealed no significant interactions between the number of pre-referral consultations and patient demographics such as age, sex, or ethnicity. However, a significant interaction was observed with cancer type, and also with referral type, although the association remained strong across all referral types.
Discussion
The findings from this National Audit of Cancer Diagnosis in Primary Care demonstrate a clear and significant association between the number of pre-referral consultations and the primary care interval. This strong correlation supports the construct validity of using the number of pre-referral consultations as a measure of the primary care interval. Whether patients were referred routinely, urgently (two-week wait), as an emergency, or via other routes, the association held, and was also observed across all studied cancer types. Notably, cancers associated with a higher proportion of patients requiring three or more consultations also tended to have longer median primary care intervals, exemplified by multiple myeloma. Conversely, cancers like breast cancer, where fewer consultations were typical, showed shorter primary care intervals.
This relationship provides valuable insights into potential strategies for reducing diagnostic delays. For instance, the considerable differences in median primary care intervals between patients requiring varying numbers of consultations suggest that reducing the number of consultations could lead to substantial improvements in diagnostic timeliness. For example, patients with five or more consultations experienced significantly longer primary care intervals compared to those with fewer consultations. These findings suggest a theoretical maximum potential for improvement in diagnostic speed if pre-referral consultations could be minimized, given the healthcare system’s capabilities and medical knowledge at the time of the audit.
While the link between pre-referral consultations and primary care interval may seem intuitive, empirical evidence supporting this association has been limited. Our findings align with existing research indicating that diagnosing cancers with non-specific symptoms (like multiple myeloma and stomach cancer) is generally more challenging for GPs compared to cancers with more distinct signs and symptoms (such as breast cancer and melanoma).
The strengths of this study include its large-scale national data, the inclusion of a wide range of cancer types, and the application of robust statistical methods. However, certain limitations must be acknowledged. There is a possibility of misclassification of consultations due to recording inaccuracies or symptom misattribution, particularly in patients with comorbidities. While the audit data was not independently validated, patient experience surveys have shown similar patterns of variation in pre-referral consultations across different cancers, lending some support to the data’s reliability. Generalizability should be considered, as participating practices may have differed from non-participating practices in terms of organizational factors and care quality. Finally, the study could not determine whether prolonged primary care intervals or high numbers of pre-referral consultations were justifiable or avoidable in individual cases.
To improve cancer diagnosis timeliness, a more liberal approach to specialist referrals and investigations for patients with non-specific symptoms could be considered. While this might increase the number of cancers diagnosed after fewer consultations, it could also lead to increased patient anxiety and healthcare costs for investigations that ultimately rule out cancer. There is growing support for enhancing GPs’ direct access to specialist diagnostic tests. Monitoring the impact of such GP-led investigations on diagnostic timeliness and resource utilization is essential and could be facilitated through primary care audit programs that include the use of diagnostic imaging and endoscopy. Point-of-care diagnostic technologies also hold promise for reducing pre-referral consultations and warrant further development and evaluation.
The study’s results underscore the importance of initiatives aimed at improving GPs’ ability to accurately assess cancer symptoms, potentially through the use of clinical decision support tools. Furthermore, raising public awareness about persistent symptoms could help shorten the intervals between consultations, thus improving diagnostic timeliness, although this may not directly reduce the number of pre-referral consultations. Future research and policy efforts should prioritize cancers that are inherently more challenging to diagnose due to non-specific symptoms (e.g., multiple myeloma, lung, stomach, and pancreatic cancers), as these are often associated with longer primary care intervals and more pre-referral consultations.
Conclusion
In conclusion, both the number of pre-referral consultations and the primary care interval are valuable and interconnected metrics for evaluating the timeliness of cancer diagnosis in primary care. Enhancing GPs’ ability to effectively evaluate cancer symptoms is a crucial priority for research and policy initiatives. Future development and evaluation of interventions should particularly target cancers that are difficult to suspect early due to their non-specific symptom presentation.
Acknowledgments
The authors express their gratitude to all primary care professionals in participating practices for their data collection efforts, and to the Cancer Networks, the Royal College of General Practitioners, and the National Cancer Action Team for their support of the audit. Access to the anonymized audit data for early diagnosis research was granted by the Audit steering group. This research was supported by the National Institute for Health Research. The views expressed are those of the authors and do not necessarily reflect the views of the NHS, the National Institute for Health Research, or the Department of Health.
