Abstract
Neurocysticercosis (NCC), a significant contributor to acquired seizures and epilepsy globally, arises from infection by the larval stage of the tapeworm Taenia solium. The advent of advanced imaging techniques like CT and MRI, alongside the development of refined diagnostic methodologies, has revolutionized our understanding and approach to Neurocysticercosis Diagnosis. These advancements, coupled with the availability of effective anti-cestode medications, have dramatically improved patient outcomes. This review delves into the critical aspects of neurocysticercosis diagnosis, exploring the evolution of diagnostic tools, the interpretation of inflammatory responses in disease progression and treatment, and the persistent challenges in achieving definitive and timely diagnoses. We address the crucial role of imaging in identifying different stages of NCC, from viable cysts to calcified granulomas, and discuss the significance of perilesional inflammation, particularly around calcified lesions, in seizure genesis. Effective neurocysticercosis diagnosis is paramount not only for individual patient management but also for broader public health strategies aimed at eradicating this preventable cause of epilepsy.
Keywords: Neurocysticercosis diagnosis, Cysticercosis, Taenia solium, Diagnosis, Seizures, Epilepsy, Imaging, Serology
Introduction
Cysticercosis, resulting from infection with the larval form of the pork tapeworm, Taenia solium, manifests when humans ingest tapeworm eggs. While the adult tapeworm resides in the human small intestine (Taeniasis), the larval form can disseminate throughout the body, leading to cysticercosis. Neurocysticercosis (NCC) specifically refers to the invasion of the central nervous system by these larvae and is a leading cause of acquired epilepsy in adults across numerous regions worldwide (Figure 1).[1, 2] Epidemiological studies estimate that NCC is responsible for approximately 29% of epilepsy cases in endemic areas globally, a figure consistent with findings from Latin America.[3, 4–7] However, the true global burden of NCC remains under-estimated due to limitations in prevalence studies.[8] Conservative estimates suggest that 1.7 to 3 million individuals worldwide suffer from epilepsy attributable to NCC.[9] NCC is prevalent in Central and South America (excluding Chile, Uruguay, and Argentina), parts of the Caribbean (notably Haiti), India, Indonesia, much of Southeast Asia, regions of China, many parts of sub-Saharan Africa (non-Muslim), areas of Eastern Europe, and regions with residual transmission in Spain.[10–15] Although transmission is minimal in the United States and most of Europe, neurocysticercosis diagnosis is frequently encountered in migrant populations originating from endemic regions.[16–19] Seizures remain the most common clinical presentation of NCC, but a substantial proportion of patients present with complex subarachnoid or ventricular disease, necessitating advanced and often prolonged treatments. Accurate and timely neurocysticercosis diagnosis is therefore crucial for effective patient management and public health interventions.
BOX 1 – LIFE CYCLE and Transmission Dynamics Relevant to Neurocysticercosis Diagnosis.
- Agent: Taenia solium, a cestode worm commonly known as the “pork tapeworm.”
- Definitive Host: Humans are the sole definitive hosts, harboring the adult tapeworm in their small intestine.
- Adult Worm Characteristics: Mature tapeworms can reach 2-4 meters in length and consist of a scolex (head) with 4 suckers and a rostellum with hooklets for attachment, a neck, and a chain of proglottids (segments) that mature distally. Ova (eggs) and/or terminal gravid proglottids are released in feces.
- Taeniasis Infection: Humans acquire taeniasis by consuming raw or undercooked pork infected with cysticerci (larval cysts). Upon ingestion, cysts evaginate in the upper small intestine, attach to the intestinal mucosa, and develop into adult tapeworms.
- Cysticercosis (Larval Form) Infection: Cysticercosis occurs in intermediate hosts, typically pigs, and also in humans. Infection happens through the ingestion of Taenia solium ova, usually via fecal-oral contamination. Once ingested, ova hatch, invade the intestines, and release oncospheres (infective embryos) that are carried via the bloodstream throughout the body. Larval cysts mature in tissues over 2-3 months. While cysts can develop in any vascularized tissue, they are most commonly found in muscles, subcutaneous tissue, and the brain, leading to neurocysticercosis. Humans, like pigs, become accidental intermediate hosts when they ingest T. solium eggs. Significantly, each proglottid can contain 30,000-50,000 ova, making human tapeworm carriers highly infectious—akin to the “typhoid Mary” of parasitology. This underscores the importance of identifying tapeworm carriers in neurocysticercosis diagnosis and control efforts.
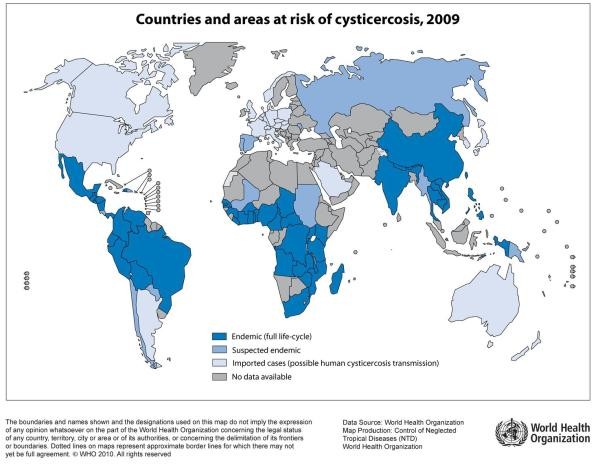
Figure 1. Global Distribution of Taenia solium Taeniasis/Cysticercosis Transmission.
Global map illustrating the distribution of Taenia solium taeniasis/cysticercosis transmission worldwide. Areas marked indicate regions where transmission is known to occur. Understanding this global distribution is crucial for clinicians in assessing risk factors when considering neurocysticercosis diagnosis, particularly in patients from or with travel history to endemic regions.
Despite significant strides in neurocysticercosis diagnosis and the availability of antiparasitic treatments, substantial gaps persist in our understanding of the parasite and host immune responses. Limitations in research include the lack of ideal clinical model infections, difficulties in maintaining the parasite life cycle experimentally, and the reliance on naturally infected humans for infectious ova. Rodent models and pig infections offer limited utility, particularly in studying seizure development. Consequently, human studies are paramount for advancing neurocysticercosis diagnosis and treatment. These studies are resource-intensive, requiring extensive clinical follow-up, advanced imaging, and specialized diagnostic tests. NCC, along with echinococcosis, remains a neglected parasitic infection, underscoring the need for increased research and resources.
Tapeworm carriers represent the primary source of infection and are at the highest risk of exposure, potentially leading to severe infections, including disseminated or encephalitic neurocysticercosis. [20–22] Family members and close contacts also face considerable risk. Environmental contamination, food, and water can serve as lower-level exposure routes, possibly explaining the high prevalence of single enhancing lesions (SELs) in regions like India.[23] Effective public health measures, coupled with improved neurocysticercosis diagnosis and treatment strategies, are essential to control and ultimately eradicate this preventable disease.
Symptomatic Disease and Neurocysticercosis Diagnosis
Types of NCC, Pathogenesis, and Diagnostic Evolution
Following ingestion, Taenia solium oncospheres disseminate via the bloodstream to various organs, including the brain. In the brain’s small blood vessels, oncospheres may or may not develop into viable cysts. Viable cysts typically form within 2-3 months. The distribution of cysts in the brain generally mirrors cerebral blood flow. Parenchymal cysts are the most common, frequently located in watershed areas between white and gray matter in cases of heavy infection. The mechanisms driving the development of large subarachnoid or racemose cysts from some parenchymal cysts remain unclear, but may involve degeneration and loss of growth control. Ventricular cysts likely originate from cysts lodging in the choroid plexus. Small ventricular cysts can move through the ventricular system or become lodged, often in the fourth ventricle, the most common site for ventricular NCC.
Clinical presentations of NCC are highly variable, influenced by the number, location, stage of development (viable, degenerating, calcified), inflammatory response, host factors, and parasite genotype.[2, 24] Neurocysticercosis diagnosis must consider the distinction between parenchymal disease (within brain tissue) and extraparenchymal involvement (subarachnoid spaces, ventricles, spine), as these differ significantly in presentation, diagnosis, and management (Table 1). However, many patients exhibit mixed disease states with multiple lesions in various stages of evolution. A single patient may present with a combination of viable parenchymal cysts with minimal inflammation, degenerating cysts with varying degrees of inflammation, calcified lesions, ventricular cysts, and hydrocephalus. The independent nature of lesion progression adds complexity to neurocysticercosis diagnosis and treatment planning.
Table 1. Diagnostic and Clinical Characteristics of Intraparenchymal and Extraparenchymal Neurocysticercosis.
| Characteristic | Parenchymal | Extraparenchymal including Spinal NCC |
|---|---|---|
| Pathology | Typical cyst or granuloma, calcification. Key for neurocysticercosis diagnosis through imaging. | Giant Sylvian cysts, ventricular cysts, basal subarachnoid NCC (“racemose”). Chronic inflammation, fibrosis. Requires different imaging approaches for neurocysticercosis diagnosis. |
| Presentation | Seizures caused by inflamed cyst or degenerating lesion, headache, focal neurological findings. Neurocysticercosis diagnosis often initiated by seizure presentation. | Mass effects, hydrocephalus from mechanical obstruction or non-communicating, headache, visual problems, cranial nerve deficits, infarcts. Neurocysticercosis diagnosis in these cases may be more complex and require broader differential. |
| Diagnosis | Imaging with characteristic cyst, calcifications. Multiple compatible lesions strengthen neurocysticercosis diagnosis. Serology supportive but variable. | Characteristic cyst in ventricles highly suggestive. Other sites less diagnostically specific unless co-exist with intraparenchymal involvement. Advanced imaging and CSF analysis may be crucial for neurocysticercosis diagnosis. |
| Treatment | Short, usual dose anthelminthics, limited corticosteroids. Response to treatment can aid neurocysticercosis diagnosis retrospectively. | May require long-term and repeated anthelminthics, long-term corticosteroids, methotrexate as possible steroid sparing. Treatment response variability impacts neurocysticercosis diagnosis follow-up. |
| Response | Usually cysticidal, cure rates slow, responds to retreatment. Imaging follow-up confirms treatment efficacy and aids in neurocysticercosis diagnosis confirmation. | Variable, relapse common. Neurocysticercosis diagnosis monitoring essential due to higher treatment failure and relapse rates. |
| Prognosis | Good, possible seizure relapses. Long-term prognosis informs neurocysticercosis diagnosis management strategies. | Not as good. Shunt obstruction is common, sizable mortality. Poorer prognosis necessitates vigilant neurocysticercosis diagnosis and management. |
The pathophysiology of symptomatic NCC largely stems from the host’s inflammatory response to degenerating cysts, cyst membranes, remnants, and residual antigens. Controlling inflammation is fundamental to mitigating morbidity and mortality. Less frequent mechanisms include mass effect and mechanical obstruction.[25] Many patients present with intricate combinations of intra- and extraparenchymal disease, lesions at diverse evolutionary stages, and varying degrees of inflammation associated with each affected area.[26] The most severe clinical manifestation typically guides the initial diagnostic and therapeutic approach in neurocysticercosis diagnosis.
Presenting symptoms are diverse and depend on the type and extent of involvement, and the intensity of inflammation. A comprehensive review of numerous publications revealed that seizures were the most common presenting symptom (78.8%), followed by headaches (37.9%), signs of intracranial hypertension (11.7%), meningitis (7.9%), cranial nerve palsies (2.8%), gait abnormalities (6%), focal deficits (16%), visual changes (5.6%), and altered mental state (4.5%).[27] Seizures are most frequent between the second and fifth decades of life and are predominantly generalized tonic-clonic seizures.[10] In children, presentations often involve single degenerating cysts or massive infections, less frequently calcifications, and rarely hydrocephalus or basal subarachnoid NCC.[28, 29] Recognizing these varied clinical presentations is crucial for prompt and accurate neurocysticercosis diagnosis.
Parenchymal NCC and Neurocysticercosis Diagnosis
Parenchymal NCC typically manifests with seizures, is generally more straightforward to treat, and carries a relatively favorable prognosis, except in cases of heavy infection. Parenchymal cysts progress through stages from viable to degenerating and may either disappear completely or persist as calcified scars.[30] (Figure 2). Neurocysticercosis diagnosis in parenchymal disease relies heavily on neuroimaging to identify cysts at different stages of evolution.
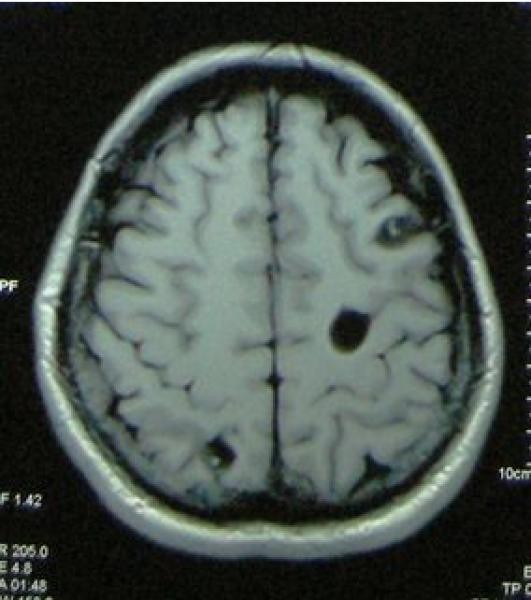
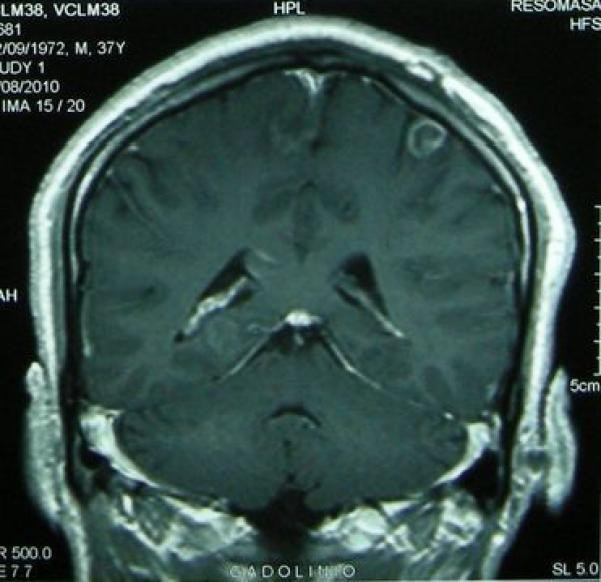
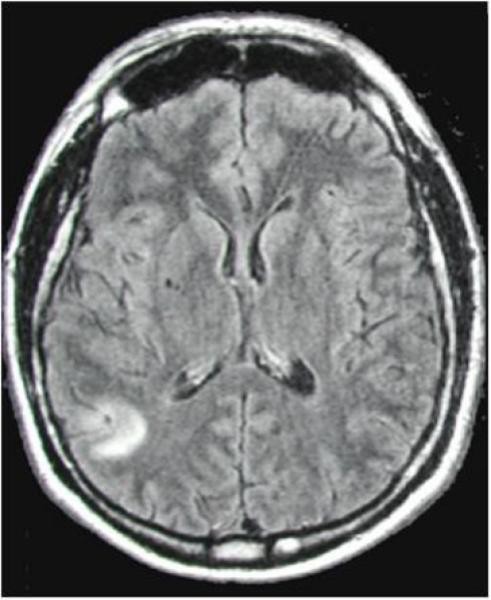
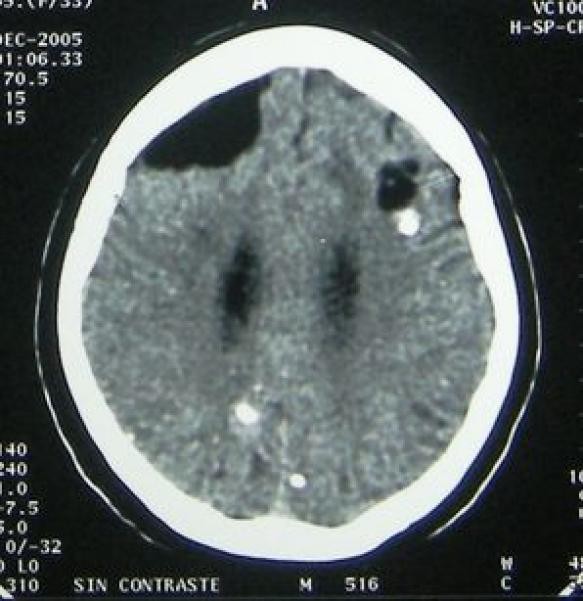
Figure 2. Stages of Intraparenchymal Neurocysticercosis as Visualized by Neuroimaging Crucial for Neurocysticercosis Diagnosis.
Figure 2a: Viable intraparenchymal neurocysticercosis cyst visualized on T1-weighted MRI. Neurocysticercosis diagnosis at this stage relies on identifying the characteristic cyst morphology.
Figure 2b: Degenerating neurocysticercosis cyst on contrast-enhanced T1-weighted MRI. Enhancement indicates inflammation, a key diagnostic feature during cyst degeneration in neurocysticercosis diagnosis.
Figure 2c: Calcified neurocysticercosis lesions visualized on non-contrast CT scan. CT is superior for detecting calcifications, a late-stage diagnostic marker in neurocysticercosis diagnosis.
Figure 2d: Perilesional edema surrounding a calcified neurocysticercosis lesion on FLAIR MRI. Edema around calcifications is diagnostically significant in patients presenting with seizures, suggesting active inflammation even in late-stage lesions and informing neurocysticercosis diagnosis and management.
Viable Cysts and Early Neurocysticercosis Diagnosis
Viable, non-degenerating cysts typically elicit minimal or no evident inflammation. The transition from a viable to a degenerating cyst, whether spontaneous or triggered by treatment, marks the initiation of a host immune response, leading to inflammation and a series of degenerative changes.[31] Neurocysticercosis diagnosis at the viable cyst stage can be challenging as these lesions may be asymptomatic and less immunogenic, potentially resulting in negative serology.
Degenerating Cysts and Active Neurocysticercosis Diagnosis
As inflammation intensifies, the cyst fluid becomes opaque. A granuloma forms around the cyst, initially as a cellular infiltrate within the cyst and subsequently as a fully formed granuloma with an inner epithelioid cellular layer. Over time, the cyst structure becomes increasingly disorganized, containing cyst remnants such as membranes and calcareous corpuscles, alongside varying degrees of inflammatory reaction. Later in the degeneration process, the granuloma shrinks, becoming nodular rather than cystic, often with contrast enhancement and sometimes surrounding edema. These lesions gradually resolve, with a reduced propensity to cause seizures compared to the active inflammatory stage.[30, 32] Neurocysticercosis diagnosis at the degenerating cyst stage is often facilitated by the presence of contrast enhancement on imaging, reflecting active inflammation, and is more likely to be associated with positive serology due to increased antigen exposure.
Single enhancing lesions (SELs) represent a common diagnostic dilemma, typically presenting with seizures. The morphology of SELs on imaging is not always definitive for NCC, and serology is frequently negative.[64] However, in endemic regions, SELs are often presumptively diagnosed as degenerating cysticerci if the lesion is less than 20mm, lacks significant edema or midline shift, no alternative etiology is apparent, and there is no subsequent lesion growth.[65] SELs generally have a favorable prognosis, but the risk of recurrent seizures increases if lesions calcify and/or develop gliosis.[33–36] Neurocysticercosis diagnosis of SELs often requires a combination of clinical context, imaging characteristics, and exclusion of other potential causes, especially in non-endemic areas.
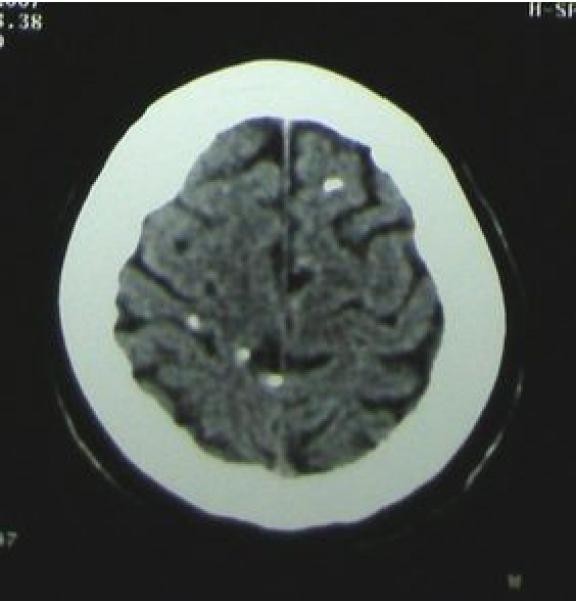
Calcified Cysts and Late-Stage Neurocysticercosis Diagnosis
Cysts may become non-visualizable, apparently resolving, or the granuloma and cyst contents may undergo hyalinization. Most calcify, becoming readily detectable on CT imaging.[37] Intermittent inflammatory flares can occur around calcified lesions, often associated with seizures.[38–40] The reasons for these flares are not fully understood but likely involve enhanced antigen accessibility and/or a lapse in host immune suppression.[32, 41, 42] The development of gliosis, likely secondary to inflammation, surrounding calcifications increases the risk of epilepsy.[43, 44] Pathological descriptions of calcifications are limited but typically reveal hyalinized, calcified lesions with variable perilesional inflammation.[30, 40, 45–48] Neurocysticercosis diagnosis in the calcified stage relies heavily on CT imaging to identify characteristic calcifications. However, it’s crucial to recognize that calcified lesions, while indicative of past infection, can still be epileptogenic, necessitating ongoing clinical management.
Extraparenchymal NCC and Complex Neurocysticercosis Diagnosis
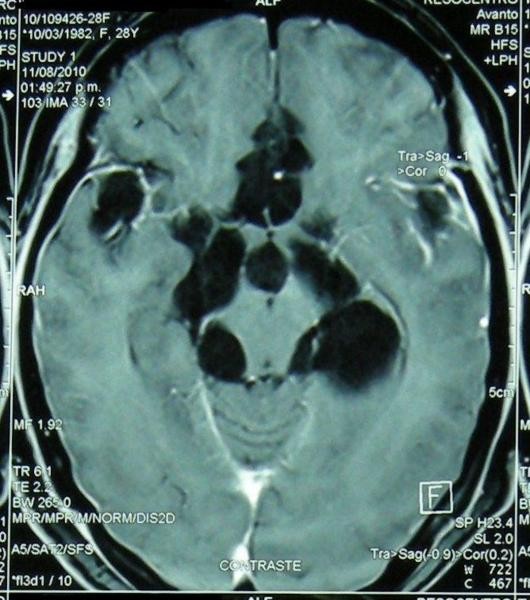
Extraparenchymal NCC, including ventricular and subarachnoid forms, is less common than parenchymal disease, more challenging to treat, and generally associated with a poorer prognosis.[49, 50] Neurocysticercosis diagnosis in extraparenchymal forms often requires advanced imaging techniques and careful clinical correlation due to the diverse and often non-specific presentations. Subarachnoid NCC can be further categorized based on location: convexity, Sylvian fissure, or basal cisterns (Figure 3), each with distinct clinical and diagnostic features.
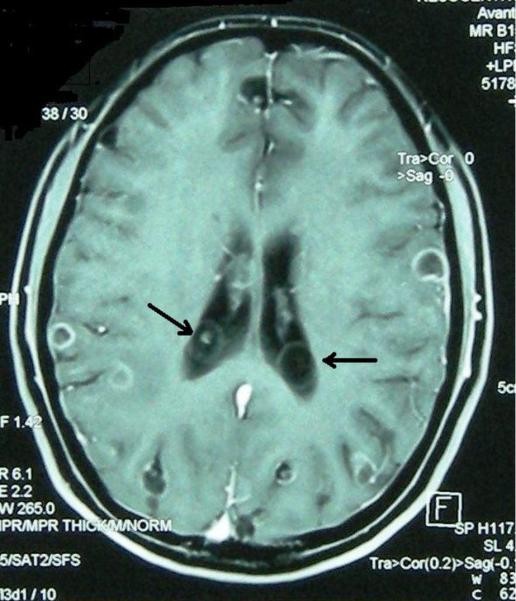
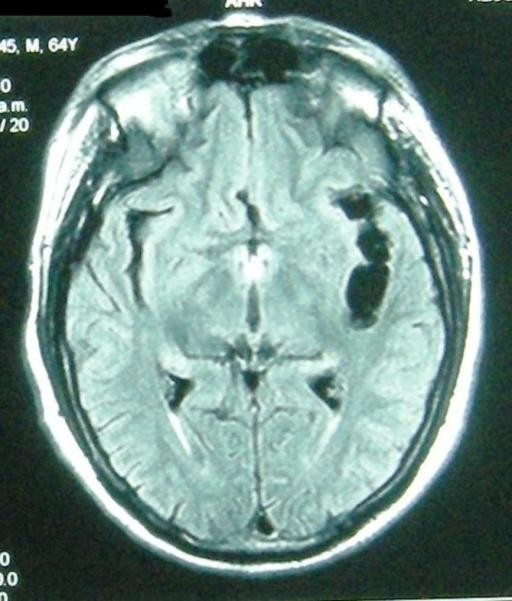
Figure 3. Extraparenchymal Neurocysticercosis: Ventricular and Subarachnoid Forms and Their Diagnostic Imaging.
Figure 3a: Intraventricular neurocysticercosis cyst (with co-existing intraparenchymal degenerating enhancing cysts) on contrast-enhanced T1-weighted MRI. Ventricular cysts are diagnostically significant and require specialized imaging techniques like FIESTA or CISS for optimal visualization in neurocysticercosis diagnosis.
Figure 3b: Subarachnoid neurocysticercosis in the brain convexity (with concurrent viable and calcified parenchymal cysts) on non-contrast CT. While CT can detect calcifications, MRI is superior for delineating subarachnoid cysts and associated inflammation, crucial for accurate neurocysticercosis diagnosis.
Figure 3c: Subarachnoid neurocysticercosis of the Sylvian fissure on FLAIR MRI. FLAIR sequences are particularly sensitive for detecting fluid-filled cysts in subarachnoid spaces and associated edema, aiding in neurocysticercosis diagnosis in these locations.
Figure 3d: Basal subarachnoid neurocysticercosis on contrast-enhanced T1-weighted MRI. Basal subarachnoid NCC (“racemose”) often requires contrast-enhanced imaging to visualize the complex, grape-like clusters of cysts and associated meningeal enhancement, critical for neurocysticercosis diagnosis in this severe form.
Intraventricular NCC and Diagnostic Challenges
Intraventricular cysts can cause mechanical obstruction of cerebrospinal fluid (CSF) flow, leading to hydrocephalus, with or without associated inflammation. Inflammation alone can also contribute to hydrocephalus if inadequately managed. The fourth ventricle is the most commonly affected location, although entrapment of lateral or third ventricles can also occur. Mobile, unattached cysts can cause intermittent positional obstruction, leading to Bruns’ syndrome (characterized by sudden nausea, vomiting, and vertigo) or a more gradual presentation with symptoms of hydrocephalus such as nausea, vomiting, headache, and gait disturbances. In pregnant women, a fourth ventricular cyst can mimic hyperemesis gravidarum but is usually accompanied by headache. Neurocysticercosis diagnosis of intraventricular NCC requires high-resolution MRI sequences like FIESTA or CISS to visualize the cyst within the ventricle. Clinical suspicion is raised in patients with obstructive hydrocephalus, particularly in endemic regions.
Subarachnoid NCC of the Brain Convexity and Diagnostic Parallels to Parenchymal Disease
Cysts located on the brain convexity typically behave similarly to intraparenchymal cysts, often presenting with seizures and progressing through the typical degenerative stages. However, they can occasionally enlarge and act as mass-occupying lesions. Neurocysticercosis diagnosis of convexity subarachnoid NCC is similar to parenchymal NCC, relying on imaging to identify cysts and monitor their evolution.
Subarachnoid NCC of the Sylvian Fissure and Complex Diagnostic Features
Subarachnoid NCC in the Sylvian fissure often presents with cyst clusters or giant cysts, resulting in mass effect. Calcifications along the middle cerebral artery suggest chronic, long-standing disease. Inflammation adjacent to brain structures, fibrosis, nerve impingement, infarcts, and, less commonly, cerebral hemorrhage contribute to the clinical manifestations.[51] Neurocysticercosis diagnosis in the Sylvian fissure involves imaging to visualize cysts, assess mass effect, and identify potential complications like infarcts or hemorrhage. The presence of calcifications along the MCA can be a distinctive diagnostic clue.
Basal Subarachnoid NCC (“Racemose” NCC) and Diagnostic Severity
Basal subarachnoid NCC, also known as “racemose” NCC, represents the most severe form of the disease due to its location and propensity for aberrant growth and proliferation.[52] This form is characterized by vesicular enlargements and continuous proliferation of parasite membranes within the subarachnoid spaces, forming a complex vesicular-membranous tissue resembling a cluster of grapes (hence “racemose”). A scolex is usually absent in these racemose cysts. Basal NCC can extensively involve the brain’s subarachnoid spaces, leading to both mass effect and intense inflammation. The inflammatory response in the arachnoid space affects meninges, foramina, adjacent brain tissue, and cranial nerves and vessels traversing these spaces, causing focal or diffuse arachnoiditis of the brain and spinal cord. Ultimately, this leads to mass effect, swelling, fibrosis, infarcts, hydrocephalus, and nerve entrapments. Chiasmal involvement can result in blindness. In some cases of basal meningitis, the cause may be unclear as distinctive vesicular membranous structures may not be evident, posing a diagnostic challenge.[53] Neurocysticercosis diagnosis of basal subarachnoid NCC requires high clinical suspicion in patients from endemic regions presenting with meningitis, hydrocephalus, or cranial nerve deficits. MRI with contrast is essential to visualize the racemose cysts and associated meningeal enhancement. CSF analysis may also be supportive, although parasite-specific findings may be variable.
Basal subarachnoid NCC can also extend throughout the subarachnoid space and involve the spinal cord.[54] While spinal involvement was once considered rare, recent studies suggest it may be present in 50-70% of patients with basal subarachnoid disease, often asymptomatically (unpublished data, T. Nash & H. Garcia, 2011). Spinal involvement ranges from arachnoiditis primarily in the cauda equina to mass effects causing paralysis or, uncommonly, numerous cysts.[55] Neurocysticercosis diagnosis should include consideration of spinal involvement in basal subarachnoid disease, particularly in patients with relevant neurological symptoms. Spinal MRI may be necessary to assess the extent of disease.
Neurocysticercosis Diagnosis: A Multifaceted Approach
The neurocysticercosis diagnosis relies on a combination of epidemiological context, clinical presentation, serological testing, neuroimaging findings, and, in some cases, pathological confirmation. While certain findings can be highly suggestive or even diagnostic, often the diagnosis is established by the overall constellation of findings, making NCC etiology the most probable explanation when other conditions are less likely. Diagnostic criteria have been proposed, but systematic validation is still needed.
Epidemiological Factors in Neurocysticercosis Diagnosis
A history of residence in or travel to endemic regions is a significant epidemiological factor in neurocysticercosis diagnosis. Most patients have lived in endemic areas for extended periods, typically in resource-limited settings with ongoing Taenia solium transmission. Conversely, the absence of significant exposure, particularly in non-endemic regions, should prompt consideration of alternative diagnoses or investigation into less conventional exposure routes. However, even minimal exposure, such as short-term travel to endemic areas or even no discernible travel history, does not entirely rule out NCC. The case of Orthodox Jews in New York infected by domestic employees from Central America with taeniasis illustrates that seemingly trivial exposures can lead to NCC.[56] Therefore, epidemiological history provides important context but should not be the sole determinant in neurocysticercosis diagnosis.
History and Physical Examination in Neurocysticercosis Diagnosis
Except for ocular cysts, which can be highly characteristic, the signs and symptoms of NCC are generally non-specific. Seizures are the most frequent presenting symptom, followed by focal neurological deficits, headache, and signs of intracranial hypertension. Seizures, often focal with secondary generalization or generalized from onset, are by far the most common manifestation. In the majority of cases, seizures are attributed to degenerating cysts or calcified granulomas, and less frequently to infarcts in patients with subarachnoid involvement.[57] Focal neurological findings are less common, non-specific, and can arise from various lesion types and mechanisms. Headaches are frequent but also non-specific, often occurring in association with seizures. Community studies have indicated a higher prevalence of NCC in individuals with migrainous headaches compared to those without headaches.[58] Hydrocephalus and intracranial hypertension are common in complex NCC and may manifest as nausea, vomiting, headache, wide-based gait, altered consciousness, and other neurological symptoms. Cognitive deficits and dementia are also frequently observed when systematically assessed.[59] While history and physical examination are crucial for initial clinical assessment, they are insufficient for definitive neurocysticercosis diagnosis and must be complemented by neuroimaging and serological tests.
Neuroimaging: Cornerstone of Neurocysticercosis Diagnosis
Central nervous system (CNS) imaging is indispensable for establishing neurocysticercosis diagnosis, determining the type and extent of disease, guiding emergency management, informing treatment choices (including immunosuppression strategies), and assessing treatment efficacy and duration. MRI is superior to CT for visualizing brain structures, anatomy, and cysts themselves, particularly viable cysts. However, CT excels in detecting calcifications. Optimal imaging protocols for neurocysticercosis diagnosis include non-contrast CT to detect calcifications and a comprehensive MRI study with FIESTA or equivalent sequences to delineate subarachnoid and ventricular disease.[60, 61]
Imaging findings can be categorized as consistent, probable, or diagnostic for NCC.[62] In the appropriate clinical and epidemiological context, the presence of roundish, 1-2 cm cysts with smooth walls and an eccentric scolex (appearing as a dot within the cyst) is considered diagnostic of NCC. The presence of multiple compatible lesions or a combination of different lesion types further strengthens the neurocysticercosis diagnosis.
MRI is particularly valuable for depicting the stages of cyst degeneration. Viable cysts appear isodense to cerebrospinal fluid (CSF) on CT and hypointense on T1-weighted and FLAIR MRI sequences. The earliest sign of degeneration is contrast enhancement, indicating blood-brain barrier disruption likely due to pericystic inflammation around a still-viable cyst. As the cyst becomes overtly opaque, its MRI appearance changes to hyperintense signal on T1-weighted and FLAIR sequences, accompanied by dense contrast enhancement.[63] These evolving imaging features are critical for staging the disease and guiding treatment decisions in neurocysticercosis diagnosis.
Single enhancing lesions (SELs) present a common diagnostic challenge because their morphology is often non-specific, and serology is frequently negative.[64] In endemic regions, SELs are conditionally considered NCC if they are ≤20 mm in diameter, no alternative etiology is identified (e.g., tuberculosis), and they do not enlarge on follow-up imaging.[65] Serial imaging is often necessary to confirm the diagnosis and exclude other possibilities in cases of SELs. Neurocysticercosis diagnosis in SEL cases relies heavily on clinical and epidemiological context, and follow-up imaging.
Calcified cysts typically appear as small, punctate, “buckshot-like” lesions on CT, although they can sometimes be larger, chunky, and irregular. A single calcification is relatively common in endemic populations but rare in non-endemic areas. Therefore, in the appropriate clinical context, a single calcification can be suggestive of NCC. Multiple calcifications are even more strongly suggestive of prior NCC infection.[32] CT imaging is essential for identifying calcified lesions and confirming late-stage neurocysticercosis diagnosis.
Serology in Neurocysticercosis Diagnosis
Numerous serological assays have been developed to detect antibodies against Taenia solium. The most extensively validated and clinically useful test is the serum Western blot assay, which utilizes a specific glycoprotein fraction of T. solium cysts. Many of the antigenic components have been identified and cloned. The Western blot is highly specific for exposure to T. solium and/or NCC disease, and is valuable for confirming the diagnosis.[66–70] However, its sensitivity is limited in patients with minimal disease, defined as those with only one or a few viable or degenerating cysts. It may also be negative in individuals with only calcified lesions.[64] In endemic regions, a negative Western blot can be helpful in ruling out NCC, but a positive result may also be seen in a significant proportion of exposed individuals without active disease.[71–73] Therefore, serology is a supportive but not definitive tool in neurocysticercosis diagnosis.
Antigen detection tests, employing monoclonal antibodies (developed against the closely related Taenia saginata) in a capture ELISA format, have also been developed. These tests are specific for current viable infection, making them particularly useful for assessing active disease. However, they are generally less sensitive than antibody assays. Antigen detection is more frequently positive in patients with subarachnoid disease compared to parenchymal disease. Antigen levels are typically higher in CSF than in serum and tend to correlate with the extent of parasitic burden.[74, 75] Serial antigen measurements may be useful in monitoring treatment efficacy and parasite clearance, particularly in complicated subarachnoid NCC, addressing the clinically relevant question of when to discontinue anthelminthic therapy.[76, 112] Antigen detection assays provide a valuable adjunct to antibody tests and imaging in neurocysticercosis diagnosis and management.
Treatment Strategies in Relation to Neurocysticercosis Diagnosis
Treatment of NCC is tailored to the individual patient, taking into account the acuity and severity of presentation, the type and extent of CNS involvement, the stage of cyst degeneration and associated inflammation, and the potential for future complications.[26, 77–79] Treatment modalities include immediate supportive measures, surgical interventions, anti-epileptic drugs (AEDs) for seizure control, corticosteroids to manage inflammation, and anthelminthic medications to target viable cysts. Randomized controlled trials for NCC treatments are limited, and thus the optimal use and benefits of various therapies are not always definitively established. However, substantial evidence and clinical experience support the use of anthelminthics for viable cysts, typically in conjunction with corticosteroids. Neurocysticercosis diagnosis, accurately staging the disease and identifying the presence of viable cysts, is paramount for guiding appropriate treatment strategies.
Symptomatic Treatment and Initial Management Following Neurocysticercosis Diagnosis
Patients with severe NCC and life-threatening complications require immediate emergency treatment. In NCC, these emergencies may include status epilepticus, intracranial hypertension (with or without hydrocephalus), infarcts, mass effect, and significant focal neurological deficits. Anthelminthic treatment is rarely the immediate priority in these critical situations and may even exacerbate the condition acutely. Prompt identification and management of intracranial hypertension are crucial. In cases with an inflammatory component, such as a degenerating cyst with edema, acute or chronic hydrocephalus, high-dose corticosteroids, along with supportive measures like shunt placement, are indicated. Symptomatic treatment addresses immediate life-threatening conditions identified through neurocysticercosis diagnosis and clinical assessment.
Analgesics and Antiepileptic Drugs
Headache is a common complaint in NCC, occurring alone or in association with seizures (post-ictal) or intracranial hypertension. Standard analgesics (paracetamol, tramadol, etc.) are typically the first-line treatment for headache, often providing effective symptom relief. AED therapy is indicated in most patients with NCC-related seizures, as these are secondary to an organic focus in the brain. Carbamazepine or phenytoin are commonly used in endemic countries due to their availability and cost-effectiveness. Formal studies comparing different AEDs in NCC-related epilepsy are lacking, but clinical experience suggests that seizure control is generally similar to that in idiopathic epilepsy. Monotherapy at standard doses is often sufficient to control seizures.[80, 81] In acute symptomatic seizures or seizures associated with significant inflammation, corticosteroids or other anti-inflammatory medications may be beneficial. AEDs are a key component of symptomatic management guided by neurocysticercosis diagnosis of seizure etiology.
Anti-inflammatory Drugs: Corticosteroids and Immunomodulation
Corticosteroids are frequently administered in NCC to control treatment-induced inflammation and to suppress inflammation during the natural course of the disease. Their use is almost universal in conjunction with anthelminthic therapy. However, the optimal regimen for corticosteroid use remains understudied, except in the context of SELs. Studies on SELs have shown that a short course of corticosteroids combined with AEDs reduces the frequency of subsequent seizures compared to AEDs alone. There is no standardized corticosteroid treatment regimen for multicystic disease. Strategies range from no corticosteroid use until symptoms arise to high-dose, prolonged administration before, during, and after anthelminthic treatment.[82] A trial comparing enhanced corticosteroid dosing (8 mg for 28 days with a 2-week taper) to standard dosing (6 mg for 10 days) in parenchymal NCC is underway to assess seizure outcomes and side effects. This study represents a significant effort to define the optimal use of corticosteroids in intraparenchymal NCC. While short courses of corticosteroids are generally safe, long-term, high-dose corticosteroid therapy, sometimes required for subarachnoid NCC and complex disease, can lead to significant side effects. Methotrexate (20 mg/week or less) has been reported as a potential corticosteroid-sparing agent.[83] Corticosteroid therapy must be carefully considered and monitored in relation to neurocysticercosis diagnosis and disease severity.
Patients commonly present with seizures associated with calcified NCC lesions and perilesional edema.[41, 42] Although high-dose corticosteroids may seem intuitively beneficial, their clinical efficacy in this context is not yet proven, and paradoxical exacerbation of the original lesion and even previously uninvolved calcifications has been observed upon corticosteroid tapering or discontinuation.[41] This “rebound” phenomenon, likely due to an overcompensation following immunosuppression, also occurs during anthelminthic treatment of viable cysts when high-dose corticosteroids are abruptly stopped. The complex interplay between inflammation, corticosteroids, and seizure control in calcified NCC warrants further investigation to optimize treatment strategies informed by neurocysticercosis diagnosis.
Anti-parasitic Treatment: Targeting Viable Cysts Following Neurocysticercosis Diagnosis
Evidence supporting the clinical benefit of anthelminthic therapy in NCC is derived from randomized, double-blinded treatment trials in single and multicystic parenchymal disease, favorable outcomes in non-randomized studies, and anecdotal evidence of clinical improvement in severe or previously lethal forms of NCC. However, data on the efficacy of anti-parasitic drugs in SELs is conflicting, with some trials showing no significant benefit.[84] The two most commonly used anthelminthics, albendazole and praziquantel, are cysticidal, leading to cyst resolution or eventual calcification.[85, 86] Despite demonstrated cysticidal efficacy, the clinical benefit of treatment was initially debated due to treatment-related exacerbations and limitations in early study designs.[84, 87] Accurate neurocysticercosis diagnosis, identifying viable cysts, is a prerequisite for considering anti-parasitic treatment.
Recent treatment studies in parenchymal NCC have shown a clinical benefit of anthelminthic therapy. A well-designed, double-blinded, randomized study comparing anthelminthic-treated versus untreated cystic NCC demonstrated a significant reduction in generalized seizures and a decrease in partial seizures in the month following treatment, supporting findings from earlier, less definitive studies.[57, 88, 89] A meta-analysis of treatment trials also confirmed clinical benefit.[90] The efficacy of anthelminthic treatment in SELs remains unclear, with variable results across studies.[91–96]
Albendazole may be superior to praziquantel at standard dosing. Uncomplicated parenchymal NCC is commonly treated with albendazole at 15 mg/kg per day in two divided doses of 400 mg with meals for 8 days to 2 weeks. However, this regimen may be underdosing some patients. Praziquantel is typically used at 50 mg/kg per day for 1-2 weeks, often with cimetidine and meals.[97] A short, high-dose praziquantel regimen (100 mg/kg in three divided doses every 2 hours), followed by corticosteroids, has shown efficacy in single-cyst NCC but its efficacy in multicystic disease is debated.[98, 99] Despite their cysticidal properties, both drugs often achieve only partial cyst eradication at standard doses. Commonly used regimens yield disappointingly low cure rates, with approximately 60% reduction in parasite load and 40% complete cyst clearance, often necessitating retreatment. Serum levels of praziquantel and the albendazole metabolite albendazole sulfoxide exhibit significant inter-patient variability and are reduced by AEDs, and praziquantel levels are also decreased by corticosteroids.[80, 81, 100, 101] A potential strategy is to treat until MRI shows initial cyst degeneration and continue treatment while tapering corticosteroids. Combined albendazole and praziquantel therapy appears safe and promising in preliminary reports.[81, 102, 103] Optimizing anti-parasitic treatment regimens, guided by neurocysticercosis diagnosis and disease monitoring, remains an area of active research.
Unlike untreated NCC, where cysts may degenerate asynchronously, anti-parasitic treatment can induce simultaneous inflammatory responses to all viable cysts. In patients with numerous cysts, this can result in widespread brain inflammation, potentially leading to severe morbidity or death. However, the ability to eliminate most viable cysts, control treatment-induced inflammation with corticosteroids, and suppress seizure activity over a relatively short period may explain the long-term reduction in seizure frequency observed after treatment. Essentially, treatment removes viable cysts, which pose the greatest risk of seizure induction upon spontaneous degeneration. Patients with multiple viable lesions require careful monitoring during treatment, and anthelminthic treatment is contraindicated in disseminated disease or ocular NCC. NCC encephalitis is primarily managed with corticosteroids. Treatment of cysts in critical locations, such as the medulla or spinal cord, requires high-dose corticosteroids and anthelminthics until lesion resolution. Anthelminthic treatment is not indicated in calcified NCC, as parasites are non-viable and only calcified cyst remnants and host inflammatory reaction persist. Treatment decisions must be carefully individualized based on accurate neurocysticercosis diagnosis and disease characteristics.
Controlled treatment trials in subarachnoid NCC are lacking. Response to anthelminthic drugs in this form is variable, typically requiring prolonged treatment and repeated assessments. Clinical, radiological, and CSF parameter changes are used to gauge improvement. A large series on medical treatment of giant Sylvian fissure cysts demonstrated relatively good responses to repeated anthelminthic courses.[51] Basal subarachnoid NCC is generally more challenging to treat, with relapses common even years after apparent stabilization. However, compared to pre-anthelminthic era outcomes characterized by severe morbidity and frequent autopsy findings, persistent care and meticulous follow-up now lead to clinical improvement and survival with reduced morbidity in many patients. Current approaches involve standard anthelminthic dosing for 2-3 months, followed by reassessment and retreatment as needed. Increased praziquantel dosing (100 mg/kg) or combined therapy may offer enhanced cysticidal efficacy in recurrent disease, although controlled trial data are still needed.[81, 104–110] Short-term high-dose praziquantel (30 mg/kg/day) has also been explored without obvious increase in side effects.[111] Serial antigen detection in serum and CSF may be valuable for monitoring treatment response and guiding duration of therapy in subarachnoid NCC.[76, 112] Management of subarachnoid NCC relies heavily on clinical judgment and ongoing monitoring informed by neurocysticercosis diagnosis.
Surgical Interventions: Addressing Complications Identified Through Neurocysticercosis Diagnosis
Surgical procedures, including neuroendoscopy, open surgery, and shunt placement, are essential in managing complicated NCC, particularly ventricular and subarachnoid forms. [113] Neuroendoscopy is useful for removing cysts that are readily accessible and causing symptoms, potentially reducing the need for prolonged corticosteroid and anthelminthic use. Randomized trials on ventricular NCC treatment are absent, but a consensus favors endoscopic removal of non-adherent ventricular cysts, if feasible. Even with successful cyst removal, hydrocephalus may still develop. Neuroendoscopic or open surgical removal of large cysts causing symptoms or likely to cause symptoms with medical treatment should also be considered.[114–118] Surgical interventions are often crucial components of NCC management, particularly in cases identified through neurocysticercosis diagnosis as having obstructive hydrocephalus or large, symptomatic cysts.
If a ventricular cyst is adherent or surgery is unavailable, high-dose corticosteroids with anthelminthics for 1-3 months or longer may be considered. Prolonged anti-inflammatory therapy (corticosteroids or methotrexate) may help suppress persistent inflammation leading to scarring and hydrocephalus. Non-obstructing lateral ventricle cysts can be managed medically, but entrapment syndromes can occur. Whether residual inflammation from remaining cysts, membranes, or antigen contributes to subsequent hydrocephalus is unknown. A large series on fourth ventricle cysts suggested medical therapy, sometimes with shunt placement for hydrocephalus, could be successful. However, as even successfully medically treated patients with prior fourth ventricle cysts may later require shunts, and long-term outcomes are uncertain, most experts recommend endoscopic or surgical removal when feasible. The advantages of surgical removal include immediate relief of obstruction, antigen source removal to control inflammation, and potentially reduced risk of obstructive and communicating hydrocephalus. [113] Surgical strategies are critical for managing specific complications identified through neurocysticercosis diagnosis.
Conclusions: Advancing Neurocysticercosis Diagnosis and Eradication
Prior to 1970, NCC was rarely recognized, infrequently diagnosed, and essentially untreatable. Currently, it is a commonly recognized infection and a major cause of seizures in endemic regions, readily diagnosed by brain imaging, and medically treatable. Despite these advancements, significant gaps remain in our understanding of the global burden of disease, the pathophysiology of NCC-related epilepsy, and the development of more effective treatments. The true global burden of NCC is underestimated, particularly in resource-limited settings where neurocysticercosis diagnosis and surveillance are limited. Optimal strategies for using cysticidal agents are still not fully defined. Cure rates remain suboptimal, and prolonged anti-parasitic therapy is often necessary for complex disease. Furthermore, anthelminthic use is a double-edged sword, inducing an inflammatory response that requires management and complicates therapy. Inflammation is the primary driver of disease manifestations, and optimal strategies for its suppression remain largely unstudied. Effective corticosteroid-sparing or -replacing agents are needed for long-term management of ventricular and subarachnoid NCC, the most severe forms of the disease, where treatment is largely based on anecdotal experience. Calcified lesions are associated with the majority of seizures in endemic populations, and perilesional edema is observed in 50% of individuals with calcified NCC experiencing seizure recurrence. The pathophysiology of seizures related to calcified lesions is unclear, but inflammation likely plays a role, suggesting that anti-inflammatory measures may be beneficial for seizure prevention and treatment in these patients. Crucially, neurocysticercosis is a preventable and potentially eradicable infection. A concerted commitment from international and national health agencies to control cysticercosis would prevent millions of epilepsy cases globally. Continued advancements in neurocysticercosis diagnosis, treatment, and prevention strategies are essential to reduce the global burden of this neglected disease.
Key Points: Advancing Neurocysticercosis Diagnosis and Management.
- Taenia solium neurocysticercosis is a prevalent cause of seizure disorders worldwide.
- Intraparenchymal NCC primarily manifests with seizures, while extraparenchymal NCC can cause mass effects and hydrocephalus, carrying a worse prognosis.
- Management includes symptomatic therapy (AEDs, steroids), and anti-parasitic drugs for viable cysts. Accurate neurocysticercosis diagnosis is crucial to guide treatment.
- Most treatment recommendations are based on expert opinion and descriptive literature, with limited controlled studies, especially beyond single degenerating lesions.
- NCC represents a valuable model for studying epilepsy pathogenesis and susceptibility.
- NCC is a preventable and likely eradicable cause of seizures and epilepsy, highlighting the importance of public health interventions and improved neurocysticercosis diagnosis strategies for control and elimination.
Biography
Dr. Nash is a Principal Investigator at the Laboratory of Parasitic Diseases, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA. His research and clinical interests encompass Giardia and giardiasis, and clinical and experimental aspects of neurocysticercosis and Taenia solium. His current research focuses on perilesional edema associated with calcified lesions as a seizure etiology, optimizing inflammation control during parasite degeneration, exploring novel corticosteroid-sparing agents, and improving treatment for complex subarachnoid neurocysticercosis. His expertise is critical for advancing neurocysticercosis diagnosis and treatment paradigms.
Dr. Garcia is a Professor at the Department of Microbiology and Director of the Center for Global Health – Tumbes, Universidad Peruana Cayetano Heredia, and Head of the Cysticercosis Unit at the National Institute for Neurological Sciences in Lima, Peru. He is also a Wellcome Trust International Senior Research Fellow and a Senior Member of the Cysticercosis Working Group in Peru. Dr. Garcia has extensive academic contributions to cysticercosis and hydatid disease. The Cysticercosis Working Group in Peru has published pivotal articles on neurocysticercosis diagnosis, treatment, and control/elimination of T. solium.
Footnotes
Review Criteria.
This review is based on information from MEDLINE searches, authors’ extensive files, and ongoing research. MEDLINE searches (1969-2011) used terms “cysticercosis”, “neurocysticercosis”, and “Taenia solium”. English and Spanish publications were reviewed. Reference selection was based on topic relevance and publication date. This rigorous review process underscores the credibility and expertise informing the discussion on neurocysticercosis diagnosis.
References
[1] Garcia HH, Gonzalez AE, Evans CA, Gilman RH. Taenia solium cysticercosis. Lancet. 2003 Oct 11;362(9391):1251-60. [PubMed PMID: 14561648]
[2] Carpio A, Escobar A, Hauser WA. Cysticercosis and epilepsy: a critical review. Epilepsy Res. 1998 May;31(1):51-71. [PubMed PMID: 9655572]
[3] Ndimubanzi PC, Carabin H, Bharath A, et al. A systematic review of the frequency of neurocysticercosis with epilepsy and seizures. PLoS Negl Trop Dis. 2010 Oct 5;4(10):e847. [PubMed PMID: 20949093]
[4] Carpio A, Placencia M, Santillan F, et al. A population-based case-control study of epilepsy and neurocysticercosis in rural Ecuador. Neurology. 1992 Sep;42(9):1619-25. [PubMed PMID: 1513516]
[5] Medina MT, Rosas-Arellano MP, Kaufer-Horwitz M, et al. Neurocysticercosis and epilepsy in Mexico. Epilepsy Res. 1990 Dec;7(2-3):139-49. [PubMed PMID: 2077970]
[6] Burneo JG, Garcia HH. Neurocysticercosis and epilepsy. J Epilepsy. 2004;17(3):129-39.
[7] Del Brutto OH, Rajshekhar V, White AC Jr, et al. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001 Feb 13;56(4):505-7. [PubMed PMID: 11222794]
[8] Murrell KD. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniasis/cysticercosis. 2005.
[9] Bernal JP, Carpio A, Moran J, et al. Epilepsy and neurocysticercosis in rural Ecuador. Epilepsia. 1999 Jul;40(7):880-6. [PubMed PMID: 10405995]
[10] Garcia HH, Del Brutto OH. Neurocysticercosis: updated review of epidemiology, pathogenesis, diagnosis, treatment, and control. Lancet Neurol. 2000 Dec;5(12):1067-77. [PubMed PMID: 17137682]
[11] Schantz PM, Wilkins PP, Brandling-Bennett AD. Neurocysticercosis in the United States: background and current status. Clin Infect Dis. 1992 Nov;15(Suppl 4):S82-9. [PubMed PMID: 1454895]
[12] Sorvillo F, Waterman SH, Richards J, et al. Cysticercosis surveillance–United States, 1990-1993. MMWR CDC Surveill Summ. 1995 Mar;44(1):1-16. [PubMed PMID: 7878448]
[13] Praet N, Speybroeck N, Manzanedo R, et al. European cysticercosis: a systematic review of epidemiological data from 1990 to 2009. PLoS Negl Trop Dis. 2009 Sep 29;3(9):e530. [PubMed PMID: 19784503]
[14] Pawlowski ZS. Taeniasis and cysticercosis. Ciba Found Symp. 1983;99:292-311. [PubMed PMID: 6415192]
[15] Garcia HH, Gonzalez AE, Gilman RH, Tsang VC. Cysticercosis in endemic regions. Lancet. 2003 Oct 11;362(9391):1295-6. [PubMed PMID: 14561658]
[16] Richards FO Jr, Schantz PM, Ruiz-Tiben E, Sorvillo F. Cysticercosis in the United States: review of an important emerging infection. Am J Trop Med Hyg. 1991 Nov;45(5):527-40. [PubMed PMID: 1959873]
[17] Jones JL, Lopez-Cervantes M, Fischer M, et al. Neurocysticercosis in Los Angeles County, California, 1991-1996. Clin Infect Dis. 2000 Apr;30(4):651-5. [PubMed PMID: 10770738]
[18] Nash TE, Mahanty S, Garcia HH. Current status of neurocysticercosis. Curr Opin Infect Dis. 2006 Oct;19(5):489-94. [PubMed PMID: 16954895]
[19] Scharf D. Neurocysticercosis in immigrants and travelers. Curr Neurol Neurosci Rep. 2008 Sep;8(5):351-7. [PubMed PMID: 18782379]
[20] Shandera WX, White AC Jr, Chen JC, et al. Neurocysticercosis among Southeast Asian immigrants to the United States. Medicine (Baltimore). 1994 Mar;73(2):101-13. [PubMed PMID: 8154448]
[21] Carpio A, Santillan F, Leon P, et al. Massive cerebral cysticercosis. Neurology. 1990 Apr;40(4):733-5. [PubMed PMID: 2325667]
[22] Agape P, Del Brutto OH, Pretell EJ, et al. Encephalitic cysticercosis: clinical and therapeutic considerations. J Neurol Neurosurg Psychiatry. 2007 Apr;78(4):418-22. [PubMed PMID: 17085562]
[23] Singh G, Kaur A, Sandhu MS, Varma S. Single small enhancing lesion on computed tomography of brain in Indian patients: aetiology and clinical significance. J Neurol Neurosurg Psychiatry. 2003 Oct;74(10):1402-4. [PubMed PMID: 14519833]
[24] Fleury A,道歉,道歉,道歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉,歉