1. Introduction
The pancreas, a vital organ nestled within the abdomen, plays a dual role, functioning as both a digestive powerhouse and an endocrine regulator. Its health is paramount to overall bodily function, making accurate diagnosis and effective treatment of pancreatic diseases critically important. Pancreatitis, an inflammatory condition of this gland, is frequently cited by clinicians as one of the most challenging and complex abdominal disorders to manage. Pancreatitis classification hinges on a triad of clinical, morphologic, and histologic parameters. Among its various forms, acute pancreatitis (AP) stands out as the most prevalent, often triggered by gallstones obstructing bile ducts or excessive alcohol consumption. The cornerstone of effective patient care lies in prompt and accurate diagnosis, coupled with a robust assessment of acute pancreatitis severity. This dual approach dictates the subsequent treatment strategy and allows for a more precise prediction of the disease’s clinical trajectory. Ultimately, this proactive stance is essential in preventing life-threatening complications, as well as mitigating organ dysfunction or outright failure. This comprehensive review aims to consolidate and present the current recommendations and guidelines for managing patients diagnosed with acute pancreatitis, with a particular emphasis on Pancreatitis Criteria Diagnosis.
Two distinct phases characterize AP: an early phase and a late phase, each further stratified by severity into mild, moderate, and severe categories [1]. A diagnosis of acute pancreatitis fundamentally requires the presence of at least two out of three cardinal features: abdominal pain, typically described as a relentless, intense epigastric discomfort frequently radiating to the back, with a sudden onset; serum lipase or amylase activity registering at least three times the upper limit of normal; and hallmark findings of acute pancreatitis detectable through imaging modalities such as ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI) [2].
Two dominant classification systems are employed for AP: the Determinant-Based Classification of Acute Pancreatitis Severity (DBC) and the Revised Atlanta Classification 2012 (RAC). Patients exhibiting persistent organ failure, categorized under severe AP, face the highest mortality risk. This underscores the critical need for early prediction and diagnosis of severe AP episodes [3]. Banks et al. [1] elucidated the Atlanta classification and the definitions of acute pancreatitis (AP), emphasizing its role in enhancing communication among clinicians, standardizing research reporting, and providing clear definitions for acute pancreatitis classification based on readily identifiable clinical and radiological pancreatitis criteria diagnosis.
Determining the underlying cause, or etiology, of AP should be a priority upon patient admission. Prompt etiological diagnosis significantly improves the chances of accurate diagnosis, enabling targeted treatment and strategies to avert complications and future pancreatitis attacks. Etiological determination relies on a thorough assessment encompassing personal and family medical history of pancreatic disease, physical examination, serum laboratory tests, and imaging studies. Concurrent with etiological diagnosis, predicting the clinical outcome of AP is crucial. This involves evaluating host risk factors, clinical risk profiles, and the patient’s response to initial therapeutic interventions [4].
Predicting AP severity and mortality involves a multi-faceted approach. This includes assessing clinical data (organ function), laboratory results, imaging findings, and employing established severity scoring systems. These evaluations should be conducted both at admission and at the 48-hour mark [3,4].
Patient management is primarily supportive, encompassing fluid resuscitation, pain management, and continuous organ function monitoring. Ensuring adequate nutritional support and providing interventional treatments, such as cholecystectomy or endoscopic sphincterotomy, or necrosectomy for necrotizing pancreatitis, are also integral components of care [3,4].
This review delves into the diagnostic methods currently available for AP, focusing on the pancreatitis criteria diagnosis used to categorize AP subtypes based on severity. It also outlines current patient management strategies for individuals suffering from this condition.
2. Classification of Acute Pancreatitis
Diagnostic approaches in AP must be tailored to the suspected cause and the patient’s clinical status. The initial diagnostic step requires confirming the presence of at least two of the three established pancreatitis criteria diagnosis. Subsequent assessments involve evaluating local complications through diagnostic imaging and systemic complications by assessing the respiratory, cardiovascular, and urinary systems. The nature and extent of these complications determine AP severity, which, in turn, guides patient management strategies [1,5].
The pancreatitis criteria diagnosis include: characteristic abdominal pain (often described as band-like), elevated serum lipase levels exceeding three times the normal threshold, and radiological evidence of pancreatitis [4,5,6]. While abdominal pain and elevated lipase are frequently observed, radiological signs are slightly less consistent. Consequently, AP diagnosis can often be established primarily based on abdominal pain and pancreatic enzyme elevation [6].
The Revised Atlanta Classification system (2012) [5], a cornerstone for defining clinical diagnosis, CT findings, and disease progression in acute pancreatitis, differentiates two primary morphological subtypes of AP: interstitial edematous pancreatitis and necrotizing pancreatitis.
This classification, which incorporates assessments of local and systemic complications alongside the presence and duration of organ failure, categorizes AP into three severity subtypes: mild AP, moderately severe AP, and severe AP [3,5]. Mortality rates vary significantly across these subtypes. Severe necrotizing AP carries a mortality risk reaching 25%, while mild edematous AP is associated with a much lower mortality rate of approximately 1%. A concerning aspect is that 20–30% of AP patients experience recurrent pancreatitis attacks, and about 10% of these progress to chronic pancreatitis (CP) [7,8,9].
Pancreatitis can lead to both local and systemic complications, each with distinct characteristics based on patient symptoms. AP can range from a mild, self-limiting condition requiring only supportive care to a severe disorder with life-threatening complications, such as respiratory and cardiovascular insufficiency or kidney failure [2,5]. Table 1 provides a detailed overview of these complications and the subtypes of acute pancreatitis, based on the 2012 Atlanta Classification.
Table 1.
Classification of acute pancreatitis based on the 2012 Atlanta Classification of Acute Pancreatitis. Explanation of crucial terms.
| Severity of Acute Pancreatitis | Characteristics |
|---|---|
| Mild acute pancreatitis | The most frequent form. No organ failure. No local or systemic complications. Usually resolves in the first week. |
| Moderately severe acute pancreatitis | Transient organ failure resolving within 48 h. Local or systemic complications without persistent organ failure. Exacerbation of co-morbid disease. |
| Severe acute pancreatitis | Persistent organ failure > 48 h. |
| Criterion of Acute Pancreatitis | Characteristics |
| Organ failure and systemic complications of acute pancreatitis | Respiratory System: Pao2/FiO2 ≤ 300. Cardiovascular System: systolic blood pressure not fluid responsive or pH Urinary System: serum creatinine ≥ 170 μmol/L. |
| Local complications of acute pancreatitis | Acute peripancreatic fluid collections. Pancreatic and peripancreatic necrosis (sterile or infected). Pancreatic pseudocyst. Walled-off pancreatic necrosis (sterile or infected). |
The RAC and DBC systems share similarities in diagnosing AP and assessing its severity. The RAC, beyond severity classification, clearly defines AP, emphasizes pain onset, and provides specific definitions for local complications, interstitial pancreatitis, and necrotizing pancreatitis [1,3]. While the RAC has three severity categories (mild, moderately severe, and severe), the DBC introduces a fourth category – critical – based on key mortality determinants: organ failure and (peri)pancreatic necrosis. Persistent organ failure coupled with infected necrosis carries the highest risk of death, necessitating intensive care unit admission and continuous monitoring. Therefore, accurate pancreatitis criteria diagnosis and predicting severe AP are paramount, particularly for identifying patients at high risk of developing complications [3].
3. Diagnosis of Acute Pancreatitis
Current recommendations for pancreatitis criteria diagnosis emphasize blood tests to measure serum lipase and amylase levels, supplemented by imaging techniques such as magnetic resonance cholangiopancreatography (MRCP), CT, and US [2]. While serum and urinary enzyme measurements are crucial for diagnosing AP, they do not reliably indicate disease severity or predict the clinical course [10,11,12].
Radiological imaging has become increasingly central to patient management due to technological advancements. Imaging techniques provide critical information for diagnosis and disease progression assessment. Ultrasonography is recommended as the initial imaging test for suspected AP, both to confirm or exclude the diagnosis and to identify potential underlying causes. MRI and CT are valuable for diagnosing local complications and detecting pancreatic necrosis or assessing AP severity. These latter two modalities are typically reserved for situations where ultrasound is inconclusive or to broaden the diagnostic picture [13]. It’s crucial to recognize that diagnosing AP is as important as identifying its etiology, which is often overlooked in initial assessments.
3.1. Laboratory Test and Indicator Enzymes
Patients presenting with sudden onset of persistent, diffuse abdominal pain or acute epigastric pain should be evaluated for acute pancreatitis. Therefore, understanding the diagnostic accuracy of serum lipase, serum amylase, urinary trypsinogen-2, and urinary amylase, individually or in combination, is crucial for effective pancreatitis criteria diagnosis [14].
An ideal laboratory test for AP should accurately diagnose the condition, facilitate early severity assessment, and identify the underlying cause. Currently, no single biochemical test fulfills all these criteria to be considered a “gold standard” for AP diagnosis and severity evaluation [10].
However, serum lipase and serum amylase remain the most relevant and widely used laboratory tests in AP diagnosis [10]. Studies indicate that serum lipase is a more reliable AP indicator than serum amylase. Urinary strip tests for trypsinogen-2 and trypsinogen activation peptide (TAP) offer reliable early AP diagnosis [15]. Other enzymes, such as pancreatic isoamylase, immunoreactive trypsin, chymotrypsin, or elastase, offer no significant advantage over lipase and are more inconvenient and costly. Measuring these enzymes is generally reserved for diagnostically uncertain cases. Crucially, neither enzyme assay directly correlates with AP severity or accurately predicts the clinical course [10,11,12]. Al-Bahrani and Ammori [15] suggest that early transient hypertransaminasemia reliably predicts biliary etiology, while serum carbohydrate-deficient transferrin is a reliable indicator of alcoholic etiology [15]. Urinary enzymes are less clinically significant in adults compared to serum enzymes but can be valuable in pediatric AP cases [16]. Nonetheless, a comprehensive understanding of all available diagnostic methods is essential.
3.2. Laboratory Tests
3.2.1. Serum Lipase and Amylase
Clinical practice guidelines from 2016 by Greenberg et al. [2,17] recommend serum lipase testing for all suspected acute pancreatitis cases due to its slightly higher sensitivity (79%) compared to other serum and urine tests (72%). Pancreatitis criteria diagnosis is confirmed when serum lipase activity is at least three times greater than the upper limit of normal.
Serum amylase testing is also used in AP diagnostics, but it holds less clinical value. Serum lipase is considered the key biochemical marker for detecting acute pancreatitis due to its earlier and more sustained elevation compared to serum amylase. Lipase levels typically remain elevated for up to two weeks, while amylase levels are elevated for a shorter period, up to five days [2,18].
Furthermore, serum lipase demonstrates slightly higher sensitivity than amylase. On days 0–1 from symptom onset, lipase sensitivity reaches 100% compared to 95% for amylase. For days 2–3, lipase sensitivity ranges from 85% with approximately 82% specificity, while amylase specificity is about 68%. This suggests lipase is particularly useful when there’s a delay between symptom onset and medical consultation [2,18].
The American College of Gastroenterology (2013) reports that measuring both serum lipase and serum amylase offers no significant advantages in treatment or cost-effectiveness [2,18]. Serum lipase is also found to be more sensitive than serum amylase in acute pancreatitis secondary to alcohol abuse [1,2,19]. Gwozdz et al. [20] compared the diagnostic values of serum and urine enzyme assays for AP recognition. Their study compared serum lipase, amylase, trypsinogen, elastase-1, 2-hour timed urine amylase excretion, and amylase and creatinine clearances. All serum tests showed similar sensitivity at admission, but serum lipase, trypsinogen, and elastase-1 tests exhibited considerably higher sensitivity than serum amylase in subsequent days. Timed urine amylase excretion did not outperform serum amylase, and the ratio of amylase to creatinine clearances showed no significant difference [20].
Rompianesi et al. [18] compared the diagnostic accuracy of serum lipase, serum amylase, urinary amylase, and urinary trypsinogen-2 in AP diagnosis. Serum lipase and serum amylase, with levels exceeding three times the normal threshold, and urinary trypsinogen-2, with values above 50 ng/mL, showed similar sensitivities (79%, 72%, and 72%, respectively) and specificities (89%, 93%, and 90%, respectively). Researchers suggest using a low threshold for one of these parameters to initiate AP treatment even if others are normal. They also emphasize considering other diseases despite normal parameters to avoid AP misdiagnosis [17].
In summary, serum lipase is the biochemically superior diagnostic assay. Lipase assays are now rapid, reliable, practical, and more specific and sensitive, without significantly exceeding amylase assay costs. Key advantages include sustained elevated levels compared to amylase, making it useful for patients presenting days after symptom onset, and greater sensitivity in alcohol-induced AP. Serum amylase assays, while also used in AP diagnosis, have lower clinical value due to lower overall specificity. Normal serum amylase typically rules out AP, except in cases of hyperlipidemia-related AP, acute exacerbation of chronic pancreatitis, or delayed amylase assessment. However, serum amylase offers advantages such as low cost, ready availability, simple automated methods, and high sensitivity [10,12,21]. Serum lipase measurement is unaffected by hypertriglyceridemia, but certain drugs (e.g., furosemide) can falsely elevate serum activity [22]. Other causes of lipase elevation include renal insufficiency, chronic pancreatitis, acute cholecystitis, or bowel obstruction [18,22]. Conversely, hypertriglyceridemia can interfere with serum amylase measurements, leading to falsely low results, which can be mitigated using lipid-clearing agents [10,18]. Abnormally low amylase levels are rarely seen but can occur in chronic pancreatitis, cystic fibrosis, smoking, obesity, and diabetes mellitus [18].
Table 2 summarizes crucial information about serum lipase and amylase for pancreatitis criteria diagnosis.
Table 2.
Crucial information concerning indicator enzymes used in the diagnosis of acute pancreatitis, based on information published in the studies of Rompianesi et al., Matull et al., and Chase et al., as well as in other publications.
| Assay | Serum Lipase | Serum Amylase |
|---|---|---|
| Origin of the enzyme | Pancreas [14] | Pancreas, salivary glands, small intestine, ovaries, adipose tissue, skeletal muscle [14] |
| The normal range of the enzyme | 5-208 U/L [18] | 30-110 U/L [18] |
| The dynamics of enzyme level | – Rise within 4–8 h; – Peak at 24 h; – Decrease to normal or near-normal levels over the next 8–14 days [22]. | – Rise within 6–24 h; – Peak at 48 h; – Decrease to normal or near-normal levels over the next 5–7 days [22]. |
| A common threshold | Three times the normal limit [1] | Three times the normal limit [1] |
3.2.2. Urinary Trypsinogen-2
Urinary trypsinogen-2 dipstick tests show higher sensitivity and specificity than urinary amylase dipstick tests [23,24]. Elevated urinary levels compared to serum levels may result from protein breakdown and peptide release during increased proteolytic activity in AP, coupled with reduced renal tubule reabsorption capacity [25].
Urinary trypsinogen-2 levels remain elevated longer in AP patients compared to both serum and urinary amylase [25]. It also differentiates between severe and mild AP more effectively than serum and urine amylase [26].
A negative urinary trypsinogen-2 test more reliably excludes AP, making it a better screening tool than serum lipase due to its higher sensitivity [25,27].
Yasuda et al. [28] confirmed that rapid urinary trypsinogen-2 dipstick tests and urinary trypsinogen-2 and TAP concentration levels are useful prognostic markers for pancreatitis criteria diagnosis. Urinary trypsinogen-2 TAP levels were significantly higher in patients with extensive extra-pancreatic inflammation (CT Grade) but not significantly higher in patients with hypo-enhanced pancreas lesions. Therefore, urinary trypsinogen-2 and TAP measurements may not be suitable for selecting patients requiring CT examination [28].
3.2.3. Urinary Amylase
Urinary amylase levels are measured using clean-catch (midstream) urine samples or 24-hour urine collections [17]. Urinary amylase shows lower sensitivity (83%) and specificity (88%) than serum amylase (85% and 91%, respectively) [25]. Urine test strips for amylase are considered useful bedside tests for AP diagnosis in patients with clinical pancreatitis [29].
The clinical utility of urinary amylase (uAm) is limited because its diagnostic ability is inferior to serum amylase (sAm). uAm has been used as a marker after endoscopic retrograde cholangiopancreatography or pancreas transplantation. The amylase creatinine clearance ratio (ACCR), an index using uAm, increases during pancreatitis but has limited diagnostic value due to low specificity and sensitivity. Terui et al. [30] reported that the uAm/uCr ratio correlates with sAm and may be an alternative to sAm for hyperamylasemia prediction. The correlation between sAm and uAm/uCr was weak in babies but significant in infants and schoolchildren, suggesting that amylase levels alone are unreliable in babies. uAm/uCr may be appropriate for various hyperamylasemia conditions after the first year of life and is not influenced by elevated sCr. In hyperamylasemia management, uAm/uCr can be used for follow-up rather than diagnosis. This suggests uAm/uCr as a potential sAm alternative. Urine samples reduce the need for blood sampling, particularly beneficial in pediatric patients, and decrease intravenous cannulation risks [30].
3.3. Ultrasonography
Gallstones and alcohol abuse account for approximately 70–80% of AP cases. Differentiating these etiologies is crucial for tailored management. Ultrasonography is the primary imaging test for suspected acute pancreatitis due to its accessibility, low cost, and lack of radiation exposure [31]. It’s also used to diagnose acute biliary pancreatitis and exclude alcohol as the primary cause. US assesses the biliary tract and detects common bile duct (CBD) stones.
Ultrasound detection of gallstones has sensitivity and specificity exceeding 95% [2,32,33,34,35,36,37], with some studies reporting 92–96% sensitivity [38,39]. However, US limitations, such as intestinal gas interference during ileus (common in AP), can be overcome by CT scans [2,32,33,34,35,36,37]. Recommended US methods for pancreas imaging include grayscale, harmonic imaging, color Doppler, and power or spectral Doppler. Pancreas sections are visualized via transverse and longitudinal upper epigastric sections, and oblique intercostal and subcostal sections, particularly for the head and tail. Transducer positioning influences visualization relative to the stomach [40].
Transgastric or subgastric sections are obtained by positioning the transducer midway between the xiphoid appendix and umbilicus. High epigastric transducer placement visualizes the pancreas through sections above the stomach antrum [40].
Optimal ultrasound windows for pancreas examination include high epigastric sections (avoiding colon gas), transgastric sections, and sections using the left liver lobe as an acoustic window [40]. If patient condition allows, 7–8 hours of fasting is necessary for optimal ultrasound examination. Stomach food mass can hinder pancreas imaging and create false images suggestive of pancreatic tumors [40].
In urgent diagnoses without proper patient preparation, ultrasound image quality may be poor and diagnosis uncertain. While US detects gallstones with high sensitivity, sensitivity drops to 65% for stones in the gallbladder infundibulum or smaller than 3 mm [40].
Endoscopic ultrasound (EUS) is a minimally invasive, highly accurate alternative for pancreas and biliary tract investigation. Figures 1 and 2 show exemplary EUS images. EUS detects gallstones with higher sensitivity than US (100% vs. 84%). EUS excels in gallbladder imaging due to proximity to the biliary system and high image resolution [38]. EUS also offers improved spatial resolution compared to MRI and CT due to probe proximity to the pancreas [41]. EUS is minimally invasive with a low complication rate, unlike ERCP. Both EUS and ERCP have 97% sensitivity for choledocholithiasis diagnosis. EUS helps select AP patients needing therapeutic ERCP, avoiding complications of diagnostic ERCP [38,39,41]. These advantages make EUS a valuable imaging technique for pancreaticobiliary disease assessment, including AP.
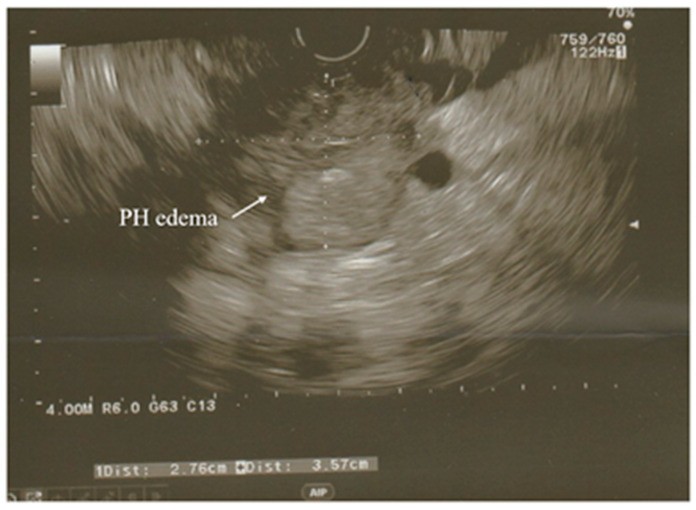
Figure 1.
Endoscopic Ultrasound (EUS) view showing acute pancreatitis with hypoechoic enlarged pancreatic head, a key diagnostic indicator. PH denotes the pancreatic head.
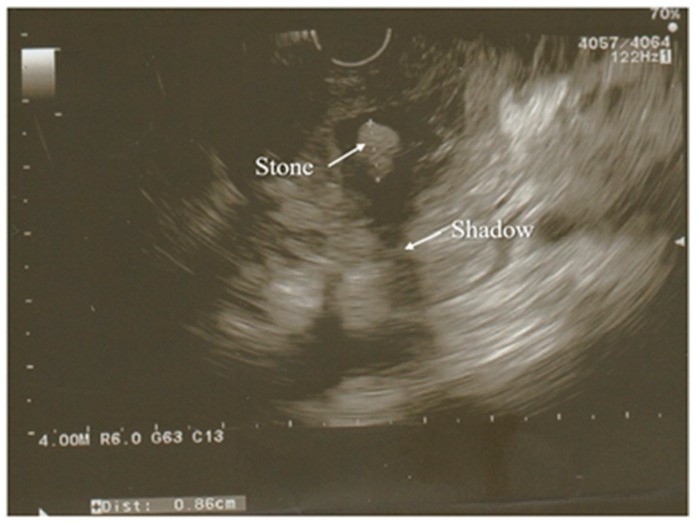
Figure 2.
Endoscopic Ultrasound (EUS) image revealing a partially calcified gallstone (with a faint shadow) in the distal common bile duct. Surrounding pancreatic tissue exhibits edema.
EUS is typically used to determine the cause of AP after the acute attack subsides, and its use during AP hospitalization is less common [38,42].
Idiopathic acute pancreatitis (IAP) and idiopathic recurrent acute pancreatitis (IRAP) pose diagnostic challenges. If etiology is identifiable in 70–90% of cases, patients are diagnosed with AP/RAP. In the remaining 10–30%, etiology remains undetermined, leading to IAP/IRAP diagnosis [38,43,44]. Etiology recognition is crucial for appropriate evaluation, early treatment, and relapse prevention. Etiology determination is vital because 50% of untreated IRAP patients experience recurrent episodes progressing to CP [38,43].
Reasons for undetermined AP etiology include biological abnormalities in early AP stages (making lipid or calcium metabolism disturbances difficult to diagnose), microlithiasis (challenging to detect with standard imaging), and pancreatic inflammation or necrosis (obscuring pancreatic cystic or solid tumors) [38,45]. EUS-based management is considered reasonable for IAP/IRAP patients. Biliary tract disease is the most frequent EUS diagnosis in IAP [38]. Key EUS indications include suspected CBD and/or gallbladder gallstones and microlithiasis. The most valid EUS indication in AP is suspected acute biliary pancreatitis when transabdominal ultrasound and CT fail to visualize biliary calculi. While EUS images the entire gallbladder, pancreas, and biliary ductal system in most AP cases, severe pancreatic necrosis, gastroduodenal anatomy variations, or rare gallbladder locations can occasionally hinder EUS examination [38].
3.4. Magnetic Resonance Imaging
MRI and MRCP are non-invasive tools for evaluating pancreatic and biliary ducts, especially the distal bile duct, which is difficult to visualize with ultrasound. They aid in diagnosing AP etiology. MRI offers advantages like no radiation exposure, no contrast agent use in non-enhanced images, no premedication, no complication risk, use during acute pancreatitis and cholangitis, and visualization of extraductal structures using standard T1-T2-weighted images. Non-enhanced MRI clearly shows necrosis areas and is safe for patients with kidney failure or allergies precluding iodinated contrast material. MRI also visualizes local complications and stages AP. Heavily T2-weighted sequences in non-enhanced MRCP, highly sensitive to fluid, can detect even small fluid amounts in mild pancreatitis [42]. MRI and MRCP non-invasively image fat or necrotic material in fluid-filled lesions and the pancreatic duct system, allowing assessment of duct integrity and communication between peripancreatic collections and pancreatic ducts [36,37].
Sun et al. [36] reported that non-enhanced MRI is more accurate and reliable than CT in assessing AP severity [36]. MRI also offers better soft-tissue contrast and avoids radiation risk compared to CT, which is important for AP patients requiring repeated follow-up exams. Non-enhanced MRI is also superior to CT in diagnosing mild AP [36].
AP diagnosis using MRI relies on detecting morphologic and peripancreatic changes. MRI effectively detects pancreatic necrosis and AP complications like abscesses, pseudocysts, or hemorrhage [28]. New MRI techniques enhance diagnosis and improve management selection. Multimodal MR images integrating MRCP, T1- and T2-weighted imaging, dynamic contrast-enhanced (DCE) MR imaging, and diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) maps are increasingly used for AP assessment before treatment planning [42]. This technique is recommended for patients with poorly visualized or normal CBD on ultrasound and elevated liver enzymes.
Romagnuolo et al.’s meta-analysis [46] reported 95% sensitivity and 97% specificity for MRCP in diagnosing biliary obstruction, a potential AP mechanism. However, MRCP’s cost limits its use for gallstone diagnosis, especially given ultrasound’s accessibility and utility for the same purpose [2,47].
3.5. Computed Tomography
Computed tomography (CT) is another key diagnostic method for acute pancreatitis. Figures 3, 4, 5, and 6 illustrate CT images in AP. CT evaluates AP extent and complications. It is a valuable diagnostic tool offering rapid scans with high spatial resolution, useful for detecting pancreatic necrosis, local complications, grading inflammation severity, and assessing AP severity [36,37,48]. CT also provides crucial information for percutaneous management [13]. Selective CT use is recommended in two scenarios: patients with suspected local AP complications (shock, peritonitis, ambiguous ultrasound) and patients with severe abdominal pain and extensive differential diagnoses confirming acute pancreatitis [2]. In essence, CT scans are indicated for patients with abdominal pain and laboratory evidence of AP, especially those with complications or inconclusive US findings.
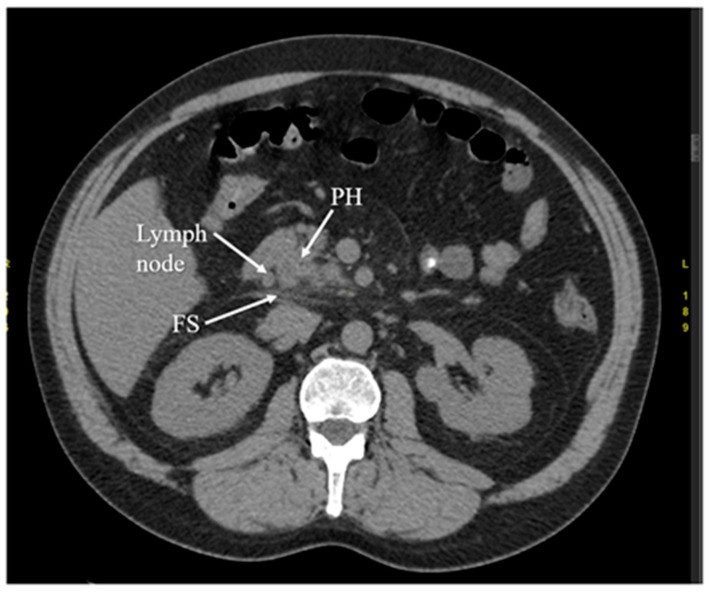
Figure 3.
Computed Tomography (CT) arterial phase image showing early acute pancreatitis with fat stranding (FS) and a single enlarged lymph node, key diagnostic features. PH indicates the pancreatic head.
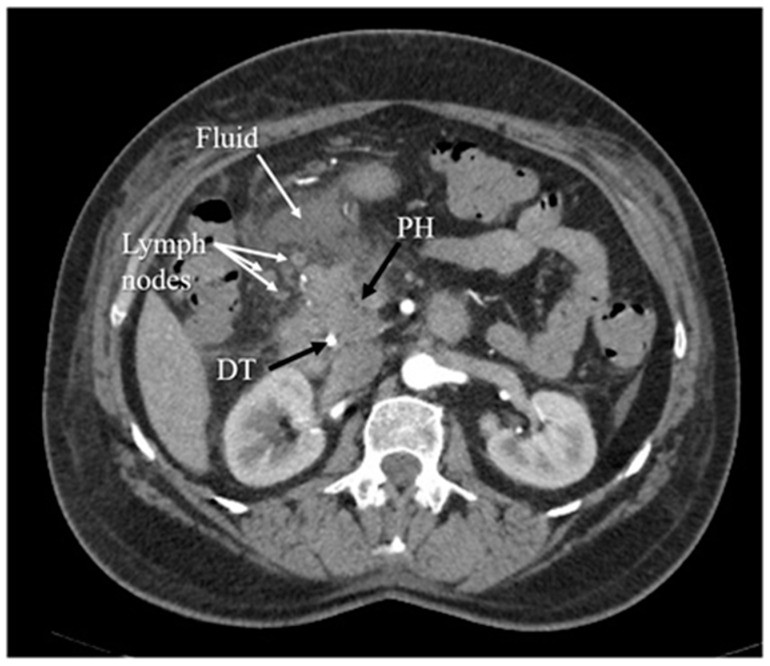
Figure 4.
Arterial phase Computed Tomography (CT) image depicting inflammation of the pancreatic head, accompanied by surrounding fluid and several enlarged lymph nodes, diagnostic signs of pancreatitis. PH denotes the pancreatic head, and DT indicates the duodenal tube.
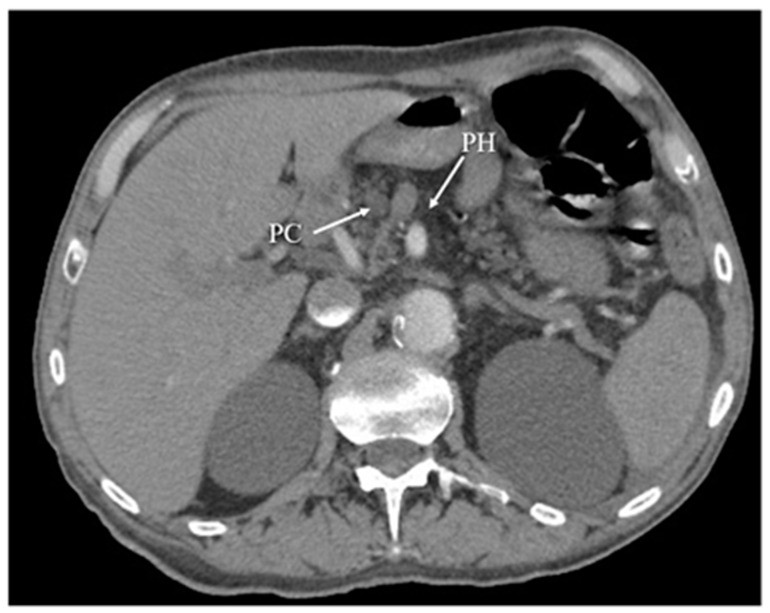
Figure 5.
Computed Tomography (CT) arterial phase image illustrating chronic pancreatitis, characterized by pancreatic head (PH) parenchyma atrophy and a pseudocyst (PC) in the same region.
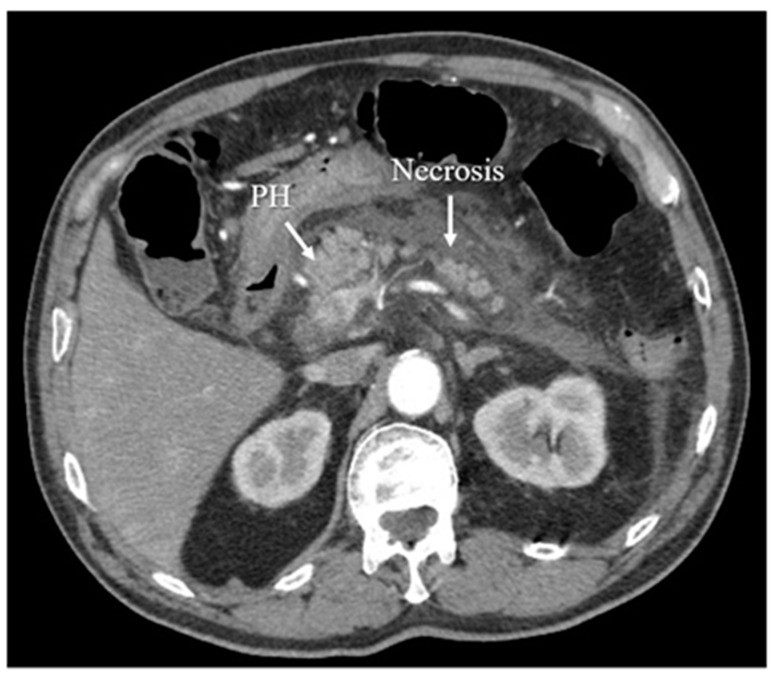
Figure 6.
Computed Tomography (CT) arterial phase image demonstrating acute necrotizing pancreatitis, a severe form of the disease requiring prompt diagnosis.
CT offers higher accuracy and sensitivity than US in diagnosis and disease extent assessment [48]. However, it has limitations: difficulty distinguishing small necrotic or fat debris within collections and radiation risk with repeated scans [36,37]. CT for local complication diagnosis is most valuable 48–72 hours after symptom onset, not at admission. After normovolemia restoration and fluid resuscitation, intravenous contrast (if no contraindications like renal failure) should be used to evaluate pancreatic necrosis.
In advanced cases, CT excludes local complications and differentiates necrotizing from interstitial acute pancreatitis. CT’s early use is limited in this differentiation, which is typically possible >3–4 days post-symptom onset. However, CT is useful in early diagnosis when broad differential diagnoses need narrowing [2,49,50]. UK guidelines for acute pancreatitis management indicate CT scans only for inconclusive biochemical and clinical features [51].
Table 3 summarizes the advantages and limitations of radiological tests used in acute pancreatitis for pancreatitis criteria diagnosis.
Table 3.
Summary of the radiological tests utilized in acute pancreatitis.
| Radiological Test | Advantages | Limitations |
|---|---|---|
| US | – Accessibility; – Low expense; – No exposure to radiation [31]; – Allows diagnosis of acute biliary pancreatitis; – Evaluates the condition of the biliary tract; – Detects biliary stones in the CBD with high sensitivity and specificity [32,35]. | – Cannot be used to diagnose alcohol overuse as a main cause of AP; – Unfavorable influence of intestinal gas occurring during ileus with bowel distension on quality of imaging; – Adverse impact of food mass in the stomach on imaging of the pancreas—disruption of precision and completeness, creation of images falsely suggesting pancreatic tumors [32,35]; – Poor quality, and therefore uncertain, diagnosis in the case of urgent management without proper preparation of the patient; – Significant decrease in the sensitivity of detection of the gallstones localized in the infundibulum of the gallbladder or characterized by the diameter less than 3 mm [40]. |
| EUS | – Minimal invasiveness; – Lower complication rate in comparison to ERCP—allows the avoidance of complications associated with diagnostic ERCP [38,39,41]; – Detection of the gallstones with higher sensitivity in comparison to US; – Close proximity to the biliary system, allowing imaging of the gallbladder better than US and providing high-image resolution [39]; – Improved spatial resolution in comparison to MRI and CT scan [41]; – Regarded as a reasonable approach for assessment of patients with IAP/IRAP; – Alternative to transabdominal ultrasound and tomographic examinations in the case of unsuccessful imaging of biliary calculi; – Imaging of the entire gallbladder, pancreas, and biliary ductal system in AP in most cases [39]. | |
| MRI | – The non-invasive evaluation of pancreatic and biliary ducts, particularly the distal bile duct, which is hard to visualize by ultrasound; – No exposure to radiation and subsequent adverse effects; – No use of a contrast agent in non-enhanced images; – Safe for the patients in the case of impossibility of receiving iodinated contrast material due to kidney failure or allergies; – No premedication; – No risk of developing complications; – Possibility to use during acute attack of pancreatitis and cholangitis; – Allows the visualization of the extraductal structures due to usage of standard T1-T2-weighted images; – Non-enhanced MRI provides clear presentation of the area of necrosis; – Visibly present local complications and stage the AP; – Allows the imaging of even a small amount of fluid in mild pancreatitis [42]; – Used to image a few fat or necrotic materials localized in a fluid-filled lesion and pancreatic duct system, which in turn allows the assessment of the duct integrity and whether collections surrounding the pancreas are in communication with pancreatic ducts [36,37]; – Non-enhanced MRI provides more precise and reliable image in assessing the severity of AP in comparison with CT; – Better soft-tissue contrast compared with CR; – Non-enhanced MRI is better in diagnosis of mild AP compared with CT [36]; – Allows the detection of pancreatic necrosis and complications of AP, such as abscesses, pseudocysts, or hemorrhage [28]; – High sensitivity and specificity of MRCP in the diagnosis of biliary obstruction [46]. | |
| CT | – Fast scans with high spatial resolution; – Allows the imaging of the necrosis of the pancreas and local complications of AP; – Enables the grading of the acuity of inflammation and the assessing of the severity of AP [36,37,48]; – Provides essential information for percutaneous management [13]; – High accuracy and sensitivity in diagnosing and providing the extent of the disease compared with US [48]; – Used to exclude local complications and distinguish necrotizing acute pancreatitis and interstitial acute pancreatitis (more than 3–4 days from the onset of symptoms); – Used in early diagnosis, in the case of the broad differential diagnosis that must be narrowed [2,49,50]. | – Difficulty to distinguish small quantity of necrotic or fat debris within one collection; – Potential radiation risk in the case of numerous follow-up scans [36,37]. |
4. Etiology of Acute Pancreatitis
Etiology determination for acute pancreatitis is crucial upon admission. This process relies on a detailed patient history (prior AP, gallstone disease, alcohol intake, medications, hyperlipidemia, trauma, recent ERCP), family history of pancreatic disease, physical examination, serum laboratory tests (liver enzymes, calcium, triglycerides), and imaging (right upper quadrant ultrasonography) [4].
Gallstones and alcohol abuse are the most common AP causes. Transabdominal ultrasound and alcohol-use history are essential for etiology determination in all AP patients because treatment and follow-up depend on the cause. For example, biliary pancreatitis patients require cholecystectomy, while alcoholic pancreatitis patients need dedicated follow-up to prevent recurrence [4,52,53,54]. Ultrasonography assesses the biliary tract and detects CBD gallstones. MRCP is recommended only for elevated liver enzymes and inadequate CBD imaging via ultrasound [2]. Alcohol-induced AP typically requires > 50 g alcohol/day, yet only ~5% of chronic alcoholics develop AP [53,55,56].
If gallstones or alcohol abuse are absent, serum triglyceride levels should be measured; levels > 1000 mg/dL suggest hypertriglyceridemia-induced AP [57,58]. Drug-induced pancreatitis is associated with medications like 6-mercaptopurine, azathioprine, isoniazid, loop diuretics, and didanosine [59,60]. Pancreatic tumors or cystic neoplasms, though rare, should be considered in patients over 40 without other obvious etiologies [53]. Post-ERCP pancreatitis is a common serious adverse event, defined as new or worsened abdominal pain and amylase at least three times normal >24 hours post-procedure, requiring admission or prolonged admission (2–3 days) [61,62]. Autoimmune pancreatitis (AIP) is a unique chronic pancreatitis form where autoimmunity against unknown autoantigens causes chronic fibro-inflammatory pancreatic responses [63,64]. AIP is classified into type 1 and type 2 based on clinical features, pathology, and IgG4 antibody responses [65]. Type 1 AIP is considered a pancreatic manifestation of systemic IgG4-related disease (IgG4-RD), characterized by elevated serum IgG4 levels and multi-organ involvement. Pathologically, IgG4-RD and type 1 AIP show massive IgG4-expressing plasmacyte infiltration and storiform fibrosis [66,67]. Type 2 AIP pathology is characterized by neutrophils, not IgG4-expressing plasmacytes [68,69]. Idiopathic AP is defined when etiology remains unknown after initial laboratory tests, transabdominal ultrasound, and CT [70].
In idiopathic AP, biliary etiology should be excluded with at least two US exams and, if necessary, MRCP and/or EUS to prevent recurrence. EUS, performed post-acute phase, assesses for occult microlithiasis, neoplasms, or chronic pancreatitis. If EUS is negative, MRCP is recommended to identify rare morphologic abnormalities, and abdominal CT should be performed. For persistent unidentified etiology, especially after a second idiopathic AP attack, genetic counseling and/or testing should be considered [3,4].
5. Assessment of Severity of Acute Pancreatitis
Determining acute pancreatitis severity is critical for patient management and prognosis. It guides timely therapeutic interventions, preventing further pancreatic and peripancreatic inflammation, complications, organ failure, and mortality [2]. Severity assessment includes clinical evaluation (cardiovascular, respiratory, renal systems), chest X-ray, BMI calculation, APACHE II score (or other severity scores), and organ failure assessment. Predicting AP complications relies on clinical severity impression, obesity, serum C-reactive protein (CRP) levels > 150 mg/L, other lab assays, Glasgow score ≥ 3, APACHE II > 8 (within first 24 hours), or persistent organ failure after 48 hours hospitalization [51]. Clinical and laboratory-based scoring systems include APACHE, Bedside Index of Severity in Acute Pancreatitis (BISAP), and Ranson’s Glasgow. Lipase and amylase levels do not predict AP severity in adults, but lipase may correlate with severity in children. Basnayake and Ratnam [11] found serum lipase activity > 7 times the upper normal limit in the first 24 hours to be a simple clinical predictor of severe AP in children.
For outcome prediction at admission, a 3-dimensional approach is recommended: host risk factors (age, comorbidity, BMI), clinical risk stratification (persistent SIRS), and response to initial therapy (persistent SIRS, BUN, creatinine) [4]. BMI, overweight, and obesity are independent risk factors for severe AP, local complications, and death. Intra-abdominal pressure (IAP) and BMI changes correlate significantly with AP severity. BMI and IAP assessment may have higher sensitivity and specificity than severity scores like APACHE-II, BISAP, CTSI, and Ranson’s score [3,71,72,73].
5.1. Serum C-Reactive Protein and Other Laboratory Assays
Helpful severity predictors, ranked by clinical value timing, include serum procalcitonin and urinary TAP and trypsinogen-2 at admission, serum interleukins-6 and -8 and polymorphonuclear elastase at 24 hours, and CRP at 48 hours [15]. CRP, an affordable and readily available marker, gradually increases with AP severity, peaking at 36–72 hours post-symptom onset, making it less useful for initial severity determination [2,74,75]. Mayer et al. [74] found serum amyloid A (SAA), an early and sensitive marker of tissue damage and inflammation, to be a better early marker of severity in the first 24 hours than CRP. CRP should be measured at admission and daily for the first 72 hours. CRP levels ≥ 150 mg/dL at admission or within 72 hours suggest acute pancreatitis and a worse clinical course [2,74].
CRP levels ≥ 150 mg/dL within 48 hours of admission aid in differentiating severe from mild disease [2]. Necrosis is associated with CRP levels > 180 mg/dL within the first 72 hours of disease onset [2]. Daily procalcitonin monitoring can non-invasively detect infected necrosis [15]. However, Farkas et al. [76] (2019) suggest neither CRP nor WBC can predict mortality or severe disease on admission, even in patients presenting within 24 hours of pain onset.
Other laboratory indicators of severe acute pancreatitis include blood urea nitrogen (BUN) > 20 mg/dL (> 7.14 mmol/L) or rising BUN, hematocrit (HCT) > 44% or rising HCT, procalcitonin, and lactate dehydrogenase (LDH) [3]. Their significance in severe AP is discussed in the management section.
5.2. Severity-of-Disease Rating Systems
Numerous rating systems exist, including Ranson’s criteria (1974), Glasgow-Imrie score (1978), APACHE II, SAPS II (1984), SOFA, CT severity index (CTSI), BISAP score (2008), and Japanese Severity score. Most scores use patient demographics, clinical features, laboratory parameters, or imaging, assessed at admission or within 48 hours. Common predictors include age, organ failure/immunocompromise, chronic disease history, temperature, blood pressure, pulse/respiratory rate, BMI, consciousness, peritonitis, acute renal failure, WBC count, hematocrit, platelet count, blood glucose, BUN, serum creatinine, AST, LDH, serum calcium, electrolytes, bilirubin, albumin, oxygen saturation, pH, base deficit, and imaging (mainly CT) [3,71].
No “gold standard” prognostic score for severe AP exists. BISAP score is considered precise and clinically applicable due to its simplicity and ability to predict severity, death, and organ failure. BISAP includes five variables: BUN > 25 mg/dL, impaired mental status, SIRS, age > 60 years, and pleural effusion on imaging [77].
Ranson’s criteria, an early scoring system, uses 11 parameters to assess AP severity, named after Dr. John Ranson. Original Ranson’s criteria use 11 parameters for alcoholic pancreatitis; modified criteria use 10 parameters for gallbladder pancreatitis [78].
Ranson’s score has limited accuracy in classifying AP severity and requires 48 hours for completion, missing the early therapeutic window. BISAP score, with admission data, accurately predicts outcome within 24 hours. Ranson’s score requires more variables, increasing diagnostic and management costs, while BISAP is cost-effective and usable in emergencies [77].
The harmless acute pancreatitis score (HAPS) rapidly identifies non-severe AP within one hour of admission. While Ranson’s score is more accurate, it takes 48 hours [79,80,81].
APACHE II score, calculable at admission and daily for 72 hours, is frequently used in ICUs globally [2]. Scores range from 0 to 71, with higher scores indicating greater severity and mortality. APACHE II values of 35–100 correspond to 85% mortality [82]. APACHE II score ≥ 8 at admission or within 72 hours suggests severe AP and worse outcomes. Higher APACHE II scores correlate with higher mortality. Mortality with APACHE II scores < 8 is approximately 83,84,85]. Kumar and Griwan [86] compared scoring systems and their utility in predicting AP severity according to 2012 Atlanta definitions. They compared APACHE II, BISAP, Ranson’s score, and modified CTSI.
Severity assessment can be done using Balthazar CTSI scoring [87] and Mortele Modified CTSI scoring [88]. Balthazar et al. [87] (1990) created CTSI by combining the original grading system with necrosis presence/extent. CTSI score did not significantly correlate with organ failure, extrapancreatic complications, or vascular complications [87]. Mortele et al. [88] (2004) developed modified CTSI to improve clinical outcome prediction. Modified CTSI is easier to calculate and correlates more closely with patient outcomes (hospital stay, surgery/intervention, infection, organ failure, death) than Balthazar CTSI, with similar inter-observer variability [88].
Balthazar CTSI scoring adds points for CT image findings and necrosis presence/extent, categorized into mild, moderate, or severe pancreatitis (Table 4). Balthazar CTSI differs from modified CTSI in CT image scoring and necrosis assessment [48].
Table 4.
Balthazar CTSI-scoring.
| Grade, Points | Characteristics |
|---|---|
| Grade A, 0 points | Normal pancreas. |
| Grade B, 1 point | Focal or diffuse enlargement of the pancreas (including contour irregularities, non-homogenous attenuation of the gland, dilation of the pancreatic duct, and foci of small fluid collections within the gland, as long as there was no evidence of peri-pancreatic disease. |
| Grade C, 2 points | Intrinsic pancreatic abnormalities associated with hazy streaky densities representing inflammatory changes in the peri-pancreatic fat. |
| Grade D, 3 points | Single ill-defined fluid collection (phlegmon). |
| Grade E, 4 points | Two or multiple poorly defined fluid collections or presence of gas in or adjacent to the pancreas. |
| The presence and extent of necrosis | Points |
| Necrosis absent | 0 points |
| 2 points | |
| 30–50% necrosis | 4 points |
| >50% necrosis | 6 points |
| Severity of AP | CTSI score |
| Mild pancreatitis | 0–3 |
| Moderate pancreatitis | 4–6 |
| Severe pancreatitis | 7–10 |
Modified Mortele CTSI scoring sums evaluated parameters, categorized similarly to Balthazar CTSI (mild, moderate, severe). Two points are added for extrapancreatic findings (Table 5) [48].
Table 5.
Modified Mortele CTSI scoring.
| Points | Characteristics |
|---|---|
| 0 points | Normal pancreas |
| 2 points | Intrinsic pancreatic abnormalities with or without inflammatory changes in peripancreatic fat. |
| 4 points | Pancreatic or peripancreatic fluid collection or peripancreatic fat necrosis. |
| The presence and extent of necrosis | Points |
| Necrosis absent | 0 points |
| 2 points | |
| >30% necrosis | 4 points |
| Severity of AP | Modified CTSI score |
| Mild pancreatitis | 0–2 |
| Moderate pancreatitis | 4–6 |
| Severe pancreatitis | 8–10 |
The revised Atlanta classification [1] categorizes severity into mild, moderate, and severe pancreatitis. Mild AP lacks organ failure and complications. Moderate AP involves transient organ failure (< 48 hours) and/or local complications. Severe AP involves persistent organ failure (> 48 hours).
Bollen et al. [89] compared CTSI and modified CTSI, and both CTSI indices with APACHE II. Modified CTSI is better than CTSI for AP severity assessment, and CTSI is better than APACHE II.
Kumar and Griwan [86] found APACHE II slightly more useful than Ranson’s score and BISAP in predicting AP severity. Raghuwanshi et al. [48] compared Balthazar CTSI and modified Mortele CTSI, both based on peripancreatic fat inflammation, intrinsic pancreatic abnormalities, fluid collection (phlegmon), and necrosis presence/extent [48].
Modified Mortele CTSI is easier to calculate and correlates more accurately with patient outcomes (hospitalization duration, surgery/intervention, infection, organ failure, death) than Balthazar CTSI. However, the revised Atlanta classification is more precise than modified Mortele CTSI and Balthazar CTSI in evaluating mortality and organ failure [48,90].
Limitations of severity scores include the need for at least 24 hours for prediction and unavailability of some parameters at admission. Early prediction of AP severity is still needed. The early achievable severity index (EASY) study is a multinational, multicenter, prospective, observational study aiming to predict severe vs. non-severe AP at admission. EASY score uses age, gender, BMI, alcohol, smoking, pain duration, blood pressure/pulse, temperature, respiratory rate, abdominal tenderness/reflex, and blood test results. Top six influential features are creatinine, glucose, respiratory rate, BUN, WBC count, and gender. A web application aids clinicians in using the model [91].
Most severity systems focus on death as an outcome. AP mortality has declined, raising questions about death as the primary outcome. IAP/APA guidelines advise using systemic inflammatory response syndrome (SIRS) to predict severe AP at admission and persistent SIRS at 48 hours. SIRS diagnosis requires ≥ 2 of 4 criteria: temperature 38 °C, heart rate > 90/min, respiratory rate > 20/min, and WBC count > 12 × 109/L or 10% bands. Persistent SIRS (> 48 hours) is associated with multi-organ failure and mortality in AP, while persistent organ failure (> 48 hours) is the main mortality determinant. SIRS (and persistent SIRS) is comparable to BISAP, APACHE II, CRP, hematocrit, procalcitonin, or BUN in predicting severe AP [3,4].
5.3. Assessment of Organ Failure
Organ failure assessment is another method to determine AP severity. Persistent organ failure (> 48 hours) despite adequate fluid resuscitation indicates severe AP [2].
Banks et al. [1] used the modified Multiple Organ Dysfunction Score [92] in their Atlanta Classification revision, using physiological indicators of failure in six organ systems to predict AP severity.
Johnson and Abu-Hilal [93] found organ failure duration in the first week of suspected severe AP strongly linked to local complications and mortality. Transient organ failure (resolving in 48 hours) was associated with lower mortality (1%) and fewer local complications (29%) compared to persistent organ failure (35% mortality, 77% complications). Organ failure resolution within 48 hours suggests a good prognosis [93].
Mofidi et al. [94] examined early SIRS importance in multiorgan dysfunction syndrome (MODS) and death in AP. Patients with SIRS > 48 hours had significantly higher MODS (Marschall Score) and mortality than those with transient SIRS (< 48 hours). They concluded SIRS is related to MODS and death in AP and is an early indicator of likely severity [94].
6. Management of Acute Pancreatitis
6.1. Supportive Care
Mild AP treatment primarily involves supportive care: isotonic intravenous fluid resuscitation, pain control, continuous vital sign monitoring, and organ function evaluation [3,51].
Fluid resuscitation should be guided by frequent hemodynamic status reassessment to avoid fluid overload. Monitored parameters include volemia and tissue perfusion indicators: hematocrit, lactate, creatinine, and BUN [3]. Fluid volume for necrosis prevention or outcome improvement is determined by patient age, weight, and pre-existing renal/cardiac conditions [95]. Aggressive fluid resuscitation effectiveness in AP lacks high-quality studies, but it likely improves pancreatic microcirculation, preventing necrosis and contributing to mortality decrease in recent decades [2,3]. Hemoconcentration is associated with increased death and pancreatic necrosis [2]. Fluid resuscitation should precede imaging.
Prompt fluid resuscitation is key to preventing systemic complications. Adequate early oxygen supplementation and fluid resuscitation until organ failure risk is excluded are associated with organ failure resolution [34,51], which correlates with low mortality [93]. Inadequate supportive care leads to organ failure, local complications, and death. Johnson and Abu-Hilal [93] found organ failure duration in the first week of severe AP affects local complication/death risk. Organ failure resolution within 48 hours is a good prognostic sign; persistent organ failure indicates higher risk of complications and death.
Maintain arterial oxygen saturation > 95% with continuous oxygen saturation measurement and supplemental oxygen. Intravenous fluids (crystalloid or colloid) maintain urine output at 0.5 mL/kg body weight. UK guidelines (2005) suggest crystalloids or colloids as needed [51], while 2019 WSES guidelines prefer isotonic crystalloids [3]. Fluid resuscitation should be monitored via frequent central venous pressure measurements. Intensive treatment is recommended until disease severity is established [51]. Alphonso Brown et al. [34] found fluid resuscitation does not prevent necrosis, but insufficient resuscitation (increased hematocrit at 24 hours) contributed to necrotizing pancreatitis in all studied patients.
Wu et al. [3] compared Ringer’s lactate and normal saline in AP fluid resuscitation. Ringer’s lactate was superior, reducing SIRS incidence by 84% and significantly decreasing CRP levels (104 mg/dL to 54 mg/dL) [46]. Ringer’s lactate’s benefits may be due to anti-inflammatory effects and better potassium level adjustment [3].
Pain relief is a clinical priority and essential supportive management for AP. Multimodal analgesia (NSAIDs, narcotics, acetaminophen) is recommended in the first 24 hours, if no contraindications exist, to improve patient quality of life [2,3,96,97]. Meng et al. [97] found no definitive data favoring specific analgesics or administration methods in AP. Perioperative acute pain management guidelines are recommended [98,99]. In non-intubated patients, dilaudid is preferred over morphine or fentanyl. Intravenous analgesia is an alternative or agonist to epidural analgesia in multimodal regimens [3], while epidural analgesia can be used for severe critical AP requiring prolonged high-dose opioids [98,99]. NSAIDs should be avoided in acute kidney injury. Patient-controlled analgesia (PCA) should be provided in all strategies [3]. Basurto Ona et al. [96] suggest opioids are suitable for AP pain treatment, reducing supplementary analgesia needs compared to other analgesics, without evidence of increased complications or adverse effects [96].
Continuous vital sign monitoring is essential due to initial treatment (fluid resuscitation, pain control, organ function assessment). High-dependency care unit monitoring is needed for organ dysfunction development. ICU admission is indicated for persistent organ dysfunction or organ failure despite sufficient fluids [3]. Transfer to a monitored unit should be considered based on three criteria: AP diagnosis with CRP > 150 mg/L, APACHE II score > 8, or organ dysfunction > 48 hours despite fluids; symptoms of current/progressing organ dysfunction (Table 6); and severe hemoconcentration (HCT > 0.500, Hb > 160) requiring aggressive fluid resuscitation [2].
Table 6.
Symptoms indicating the current or progressing organ dysfunction in the course of acute pancreatitis, the presence of which is a criterion for consideration of admission to a monitored unit.
| Impaired Organ | Symptoms |
|---|---|
| Respiratory | 1. Pao2/FiO2 ≤ 300 2. Respiratory rate > 20 breaths per min |
| Cardiovascular | 1. Need for vasopressors in the case of non-fluid-responsive patients 2. Hypotension, despite aggressive fluid resuscitation, defined as systolic blood pressure (sBP) 40 3. pH |
| Renal | – 4.Urine output – 5.Increase of ≥ 26.5 μmol in serum creatinine over 48 h – 6.≥1.5-fold increase in serum creatinine over 7 days |
Patients meeting one or more criteria with BMI > 30 (Asian populations > 25) require thorough monitoring and lower threshold for monitored care unit transfer due to reports of worse disease course in obese patients. Severe AP increases death risk, and organ failure is seen in deceased patients. Organ failure > 48 hours in the first week poses the highest death risk [100]. Suspected severe AP patients should be carefully monitored, preferably in high-dependency units, with ICU supportive care for organ dysfunction or failure [51]. Nathens et al. [101] found intensivist care or consultation in closed ICUs resulted in shorter ICU stays and lower mortality in critically ill severe AP patients compared to those without specialized intensivist care [101].
AP complications include organ failure. AP management guidelines [3] recommend mechanical ventilation for respiratory failure (Pao2/FiO2 ≤ 300 and respiratory rate > 20 breaths/min) [1,2]. No specific issues exist in treating respiratory failure in AP patients. If high flow nasal oxygen or CPAP are ineffective for tachypnea and dyspnea, mechanical ventilation is needed. Non-invasive and invasive techniques can be used. Invasive ventilation is obligatory if bronchial secretion clearance is ineffective and/or patient fatigues or is predicted to fatigue. Lung-protective strategies are indicated with invasive ventilation [3]. Hypoxia partially explains tachypnea and dyspnea. Pleural effusion, intra-abdominal hypertension, or pain can cause these symptoms despite adequate oxygenation. Pulmonary edema may occur post-fluid resuscitation, exacerbated by increased systemic permeability [28,102].
Intra-abdominal pressure control is another key supportive care element. Systemic inflammation-induced increased systemic permeability and treatments like vasoactive drugs or fluid resuscitation aggravate intra-abdominal hypertension and intestinal failure. Excessive sedation can worsen intestinal dysfunction, increasing intra-abdominal pressure. Deep sedation and paralysis are mandatory before surgical abdominal decompression to limit intra-abdominal hypertension if non-operative treatments (percutaneous intraperitoneal fluid drainage) are insufficient. Restrict vasoactive drugs, fluids, and sedation to achieve resuscitative goals at lower normal limits. Limit ICU medications when side effects outweigh benefits [3,103].
6.2. Improvement of Laboratory Parameters
Severe AP, defined by persistent organ failure (> 48 hours), has a 20–50% mortality rate [1]. Early severity assessment is crucial for therapeutic strategy determination, as effective treatment can significantly reduce mortality in severe AP. Invasive and non-invasive methods assess AP severity, including scoring systems, radiological imaging, and biochemical parameters. Laboratory parameters like albumin, glucose, HTC, TG, CRP, procalcitonin, creatinine, BUN, and eGFR should be constantly monitored and corrected if possible during patient management [3,104].
Hypoproteinemia is observed in AP patients. Low albumin may be a key factor in AP pathogenesis. Li et al. [105] found low serum albumin at admission independently associated with persistent organ failure in severe AP, suggesting albumin as a rapid assessment tool for persistent organ failure in AP.
High glucose level is a Ranson’s criteria parameter. This and other severity scores predict AP mortality, associated with AP severity. Glycemia is assessed at admission. Ranson’s scoring considers blood glucose > 200 mg/dL significant, while modified Ranson’s scoring considers > 220 mg/dL [3,78,81,86,106].
Serum triglyceride and calcium levels should be measured if gallstones or significant alcohol history are absent. Triglyceride levels > 11.3 mmol/L (1000 mg/dL) indicate hypertriglyceridemia-induced AP, necessitating lipid-lowering therapy [3,107].
Urea > 20 mg/dL independently predicts mortality, while hematocrit > 44% is an independent risk factor for pancreatic necrosis. Procalcitonin levels can predict infected necrosis in patients with confirmed pancreatic necrosis. Procalcitonin ≥ 3.8 ng/mL within 96 hours of symptom onset indicates pancreatic necrosis with 93% sensitivity and 79% specificity. Procalcitonin is considered the most sensitive lab test for pancreatic infection. Low serum values are strong negative predictors of infected necrosis [108,109,110,111,112]. Serum LDH level at admission can predict severe AP, death, and ICU admission but should be measured with other laboratory parameters [106].
Persistent organ failure includes kidney insufficiency, decreasing eGFR and increasing creatinine due to renal injury and pancreatic necrosis [113]. Lipinski, Rydzewki, and Rydzewska [114] found serum creatinine and eGFR measured at admission and 48 hours later significantly associated with pancreatic necrosis. Serum creatinine and eGFR on day 1 were good predictors of fatal outcome. Pancreatic necrosis and mortality were significantly higher in patients with elevated serum creatinine and low eGFR. They suggested serum creatinine and eGFR as useful mortality and pancreatic necrosis indicators at admission.
In conclusion, low albumin, high glucose, high HCT, high triglycerides, and low eGFR should be constantly monitored and corrected if possible.
6.3. Nutrition
Acute pancreatitis pathogenesis involves premature activation of pancreatic proteolytic enzymes within pancreatic ducts, causing pancreatic autodigestion. Bowel rest was historically expected to reduce pancreatic inflammation. However, recent RCTs show early oral/enteral feeding in AP patients does not cause adverse effects and may reduce pain, food intolerance, and opioid use [2].
World Association guidelines and the Santorini consensus consider enteral nutrition safe in AP [3], but it doesn’t improve mild pancreatitis, and dietary restrictions are unnecessary in mild cases. Artificial feeding can provide long-term nutritional support and prevent complications in AP. Nausea inhibits oral food consumption in severe AP [51]. Acute inflammation disrupts the intestinal mucosal barrier, making enteral nutrition recommended to prevent intestinal failure and infectious complications. Enteral nutrition preserves the intestinal mucosa barrier, preventing bacterial displacement that could colonize pancreatic necrosis. It also reduces inflammatory response stimuli, oxidant stress, and systemic endotoxin exposure [3,51,116].
Windsor et al. [18] compared enteral to parenteral nutrition and concluded enteral feeding reduces acute phase response and AP severity and improves clinical outcome, without CT scan changes in pancreatic injury. Enteral nutrition is clinically beneficial, safer than parenteral nutrition, and causes fewer septic complications [18,51]. Continuous infusion is more common than cyclic or bolus administration [3].
Standard diet is recommended for mild AP patients at admission. If symptoms (nausea, vomiting, abdominal pain, ileus) prevent oral diet tolerance, patients can self-adjust diet, withhold food/fluids, or follow a regular diet [2]. Eckerwall et al. [117] showed oral nutrition at admission reduced hospitalization from 6 to 4 days in mild AP, compared to withholding food/fluids. Early feeding benefits are observed when started within the first 48 hours. Oral feeding should commence at admission if tolerated or within 24 hours [118].
During mild pancreatitis recovery, patients initially fast, then refeed orally with a clear liquid diet (CLD) to prevent gastrointestinal events, gradually transitioning to soft solids. Hospital discharge depends on dietary change effectiveness and solid food tolerance. Jacobson et al. [119] (2007) and Sathiaraj et al. [120] (2008) showed initiating oral nutrition with a low-fat solid diet (LFSD) instead of CLD is safe post-mild AP and provides more calories. Sathiaraj et al. [120] found this reduces hospitalization, while Jacobson et al. [119] found no such link. Low-fat diet superiority over regular diet is unproven [2].
Enteral feeding in predicted mild pancreatitis can resume once inflammatory markers improve and abdominal pain decreases. No need to delay enteral feeding until lab abnormalities or pain fully resolve. Immediate oral refeeding with a normal diet is safe in predicted mild pancreatitis, accelerating recovery and shortening hospital stays without adverse gastrointestinal events. Starting with liquid or soft diets is unnecessary; full solid diet feeding is possible in predicted mild pancreatitis [4,117,121].
In severe acute pancreatitis, enteral nutrition should be introduced within 48 hours of admission [2]. Kalfarentzos et al. [122] indicated early enteral nutrition is superior to parenteral nutrition in severe AP due to fewer complications, lower septic complication risk, and lower procedure cost. Enteral nutrition is preferred over parenteral nutrition in AP and severe AP. RCTs show enteral nutrition reduces mortality, local septic and other complications, systemic infection, surgical interventions, and multiorgan failure compared to parenteral nutrition, and shortens hospitalization [10,24].
Similar results are achieved when enteral nutrition is administered within 48 hours of admission [123]. Total parenteral nutrition should be avoided in acute and severe acute pancreatitis. Partial parenteral nutrition can be considered if enteral feeding is incompletely tolerated to ensure caloric and protein needs are met [3]. Gupta et al. [124] found no significant clinical benefits in recovery time comparing early enteral to parenteral nutrition. Enteral nutrition may be limited by ileus. Parenteral nutrition becomes mandatory if ileus lasts > 5 days [51].
Both gastric and jejunal nutrition are safe, but nasojejunal tube is not superior to nasogastric tube. Feeding administration should not be delayed for nasojejunal tube insertion [3].
Petrov et al. [125] found no mortality or food intolerance changes comparing nasogastric and nasojejunal enteral nutrition. Chang et al. [126] found no significant differences in pain, diarrhea, tracheal aspiration, energy balance, or mortality between nasogastric and nasojejunal tube-fed patients. Early nasoenteric tube nutrition within 24 hours in necrotizing pancreatitis, compared to oral diet at 72 hours, did not reduce infection or death incidence [3]. Nasogastric nutrition is effective in up to 80% of patients. Caution is needed with nasogastric nutrition in incompletely conscious patients due to aspiration risk [51].
Various formulations are used in pancreatitis, but no evidence supports advantages of standard, elemental, partially digested, or immune-enhanced formulations [51]. Petrov et al. [127] and Sun et al. [99] found insufficient evidence to support clinical benefits of immune-enhanced, semi-elemental, and probiotic enteral feeds in severe AP management [99,127].
6.4. Pharmacological and Antimicrobial Treatment
No specific drug therapy is proven effective for acute pancreatitis [3,51]. Broad randomized studies show no clinically significant benefits from anti-inflammatory agents (lexipafant) and antiproteases (gabexate), or antisecretory agents (octreotide) [128,129,130]. Prophylactic antibiotics are not recommended for mild or severe acute pancreatitis [2,3]. Antibiotics are always advised for infected severe acute pancreatitis, the most serious local complication [3,51].
Gram-negative bacteria dominate pancreatic infections, but Gram-positive bacteria, anaerobes, and fungi can also be present [131]. Common fungi include Candida albicans, Candida tropicalis, and Candida krusei [3]. Antibiotics with proven penetration into pancreatic necrosis are recommended for infected necrosis. Empirical antibiotic therapy should cover aerobic and anaerobic, Gram-positive and Gram-negative microorganisms [3].
Intravenous aminoglycosides do not penetrate the pancreas sufficiently [132]. Acylureidopenicillins and third-generation cephalosporins penetrate moderately, achieving MIC for most Gram-negative bacteria in pancreatic infections. Piperacillin/tazobactam is effective against Gram-positive anaerobes [133]. Quinolones (ciprofloxacin, moxifloxacin), carbapenems, and metronidazole have good pancreatic tissue penetration and anaerobic bacteria effect [3,134]. Quinolones should be reserved for beta-lactam allergy due to high bacterial resistance. Carbapenems should be reserved for critically ill patients due to carbapenem-resistant Klebsiella pneumoniae [3].
Fungal infections are serious AP complications, increasing morbidity and mortality. Fungal infection prevention is not recommended due to insufficient studies [135].
Pain management is essential in AP. No specific analgesics are used; perioperative acute pain management guidelines are recommended [3].
6.5. Surgical and Operative Treatment
ERCP is used to treat acute gallstone pancreatitis but not routinely. ERCP indications in acute gallstone pancreatitis include cholangitis and common bile duct obstruction [3].
Intervention (percutaneous or endoscopic drainage) is recommended for suspected or confirmed infected necrotizing pancreatitis with clinical deterioration. Walled-off pancreatic necrosis (WOPN), a mature, encapsulated necrosis collection with an inflammatory wall, often occurs > 4 weeks post-onset. Intervention for necrotizing pancreatitis should start after necrosis is walled-off, approximately after 4 weeks. Indications for intervention > 4 weeks post-onset include symptomatic or growing pseudocyst, disconnected duct syndrome, ongoing organ failure without infected necrosis, and ongoing gastric outlet, biliary, or intestinal obstruction due to large WOPN. Percutaneous or endoscopic drainage of pancreatic collections > 8 weeks post-onset is indicated for ongoing pain and/or discomfort [3].
Surgical strategies are considered if percutaneous or endoscopic interventions fail. Surgical treatment indications include abdominal compartment syndrome, bowel ischemia or acute necrotizing cholecystitis during AP, bowel fistula into peripancreatic collection, and acute ongoing bleeding (if endovascular approach fails). Surgery is also used as a step-up approach after failed percutaneous or endoscopic procedures with the same indications [3]. Eastern Association for the Surgery of Trauma [136] showed later surgery improves survival. Surgical intervention > 4 weeks post-onset is recommended to reduce death likelihood [3], as necrosis becomes more separated from vital tissue, reducing vital tissue damage, bleeding, and improving necrosectomy effectiveness in late surgery [3].
International Association of Pancreatology (IAP) recommendations [137] state mild AP is not a surgical indication. Cholecystectomy is recommended for mild gallstone-associated AP, preferably during the same hospitalization after patient stabilization. Surgical treatment of acute gallstone pancreatitis involves cholecystectomy with operative cholangiography [4,137,138]. Surgery is not used in most AP patients, but many eventually undergo cholecystectomy [51]. IAP guidelines recommend cholecystectomy to prevent gallstone-associated AP recurrence [137]. Same admission cholecystectomy is recommended if ERCP and sphincterotomy are performed to avoid other biliary complications. Cholecystectomy should be postponed until fluid collections subside or stabilize and acute inflammation resolves if peripancreatic fluid collections develop in acute gallstone pancreatitis [3].
Early surgery within 14 days of onset is only recommended for specific indications in necrotizing pancreatitis [51]. Minimally invasive transgastric endoscopic necrosectomy may be considered for disconnected pancreatic duct patients and selected WOPN cases. Percutaneous drainage is the first-line treatment for infected pancreatic necrosis, allowing surgical treatment postponement and potentially resolving infection completely in 25–60% of cases [3]. Open abdomen surgical decompression is effective for abdominal compartment syndrome in severe AP unresponsive to conservative intra-abdominal hypertension/compartment syndrome management. Open abdomen procedure should be avoided if other strategies can alleviate severe intra-abdominal hypertension in severe AP [3].
Surgical strategies should be considered if endoscopic procedures fail to improve patient condition [3].
7. Prevention of Acute Pancreatitis
Identifying AP etiology at admission is crucial for optimal and specific therapy. Early ERCP is best for biliary AP with cholangitis; lipid-lowering therapy for hypertriglyceridemia-induced AP; pancreatic stent placement for obstruction-evoked AP; and steroid therapy for autoimmune pancreatitis [107].
Zádori et al. [107] found 5% of patients left the hospital after first/second AP attacks without imaging, and 25% had no biliary AP diagnostic workup (lab tests). Etiology screening was most insufficient for lipid-induced pancreatitis (71–76%). Additional diagnostic workup for idiopathic AP after index admission was lacking: 91% lacked biliary, anatomic, or cancer etiology search via EUS/MRCP; 98% lacked autoimmune AP search; 99% genetic AP; and 94% virus-induced AP after the first attack.
IAP/APA guidelines are insufficiently implemented in daily practice. AP etiology remains unclear in almost 25% of cases due to insufficient diagnostic workup or unknown etiologies. Idiopathic etiology causes ~40% of fatal AP cases, emphasizing etiology determination significance. Etiology definition is crucial for index AP and preventing recurrent/chronic pancreatitis [107].
Biliary etiology should be excluded in idiopathic AP with at least two US exams to prevent recurrent pancreatitis. EUS, post-acute phase, assesses for occult microlithiasis, neoplasms, or chronic pancreatitis. If EUS is negative, MRCP identifies rare morphologic abnormalities. Genetic counseling/testing is considered for persistent unidentified etiology, especially after a second idiopathic AP attack, to diagnose hereditary pancreatitis or understand AP genetic risks [3,4,107].
AIP diagnosis is based on clinical and radiological features, serological parameters, and pathological findings. International Consensus Diagnostic Criteria for Autoimmune Pancreatitis (ICDC) for type 1 AIP use five cardinal features: (1) pancreatic parenchyma imaging (CT/MRI) and duct (ERCP/MRCP) features; (2) serum IgG4 level; (3) other organ involvement; (4) pancreatic histopathology; and (5) steroid therapy response. Type 2 AIP diagnosis uses four cardinal features from type 1, excluding serology [139,140].
Márta et al.’s meta-analysis [141] suggested lactated Ringer’s and indomethacin combination to prevent post-ERCP pancreatitis. Aggressive hydration with indomethacin is more effective than single therapy and all other interventions, suggesting a one-hit-on-each-target therapeutic approach using easily accessible aggressive hydration and indomethacin for all average and high-risk patients without contraindications.
8. Conclusions
This review summarizes key information on acute pancreatitis diagnosis, evaluation, and treatment. While numerous laboratory tests and imaging methods exist, only some are diagnostically useful; others assess disease severity. Early supportive care and proper nutrition are crucial. Drug treatment is non-specific, focused on pain management according to current guidelines. Surgical treatment emphasizes minimally invasive percutaneous or endoscopic procedures, with open abdominal surgery reserved for exceptions. This review provides a useful summary of acute pancreatitis information for medical students and young clinicians, particularly regarding pancreatitis criteria diagnosis.
Author Contributions
J.W.—project development, data collection and management, data analysis, and manuscript writing; N.Z.—data analysis and manuscript editing; M.P.—data analysis and manuscript editing; R.S.T.—data analysis and manuscript editing; J.D.-R.—data analysis and manuscript editing; Ł.O.—data collection, data analysis, and manuscript editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Please contact authors for data requests (Łukasz Olewnik—email address: [email protected]).
Conflicts of Interest
The authors declare that they have no competing interests.
Funding Statement
The authors have no financial or personal relationship with any third party whose interests could be positively or negatively influenced by the article’s content. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact authors for data requests (Łukasz Olewnik—email address: [email protected]).