Introduction
Parotid gland tumors, while representing a small fraction of head and neck neoplasms (3-6%), are a significant clinical entity with a global incidence ranging from 0.4 to 13.5 per 100,000 individuals annually 1. The majority, approximately 80%, are benign, with pleomorphic adenomas being the most prevalent 2. Benign tumors typically manifest in the fourth decade of life, whereas malignant counterparts tend to peak in the sixth and seventh decades 3–5. There’s a slight male predominance for benign tumors (male:female ratio = 1.1:1), while malignant tumors show a more even sex distribution 1.
Clinically, parotid tumors often present as slow-growing, painless masses. However, symptoms like pain, facial nerve palsy, skin ulceration, rapid growth, otalgia (ear pain), and cervical lymph node enlargement are red flags for potential malignancy 6 7.
Fine-needle aspiration cytology (FNAC) is a common initial diagnostic step due to the parotid gland’s accessibility. FNAC boasts a high specificity (0.97) and sensitivity (0.80) 8, with minimal risk of tumor cell dissemination 9. Yet, it yields inconclusive results in about 6% of cases due to insufficient sample size or deep lobe tumor location 10 11.
Preoperative imaging, particularly Magnetic Resonance Imaging (MRI), is crucial for surgical planning, aiding in tumor localization and malignancy assessment. Benign tumors may be managed with superficial parotidectomy or extracapsular enucleation, while malignant lesions often necessitate total parotidectomy, potentially involving facial nerve sacrifice 12 13. Ultrasound (US) is a primary imaging modality for lymph node and soft tissue evaluation in the head and neck, including salivary glands 14. However, US has limitations in visualizing the deep parotid lobe due to mandibular shadowing and in assessing facial nerve involvement, retropharyngeal/deep neck adenopathies, and intracranial or skull base extension 14–16. MRI emerges as the preferred method for parotid tumor evaluation because of its superior soft tissue contrast, deep lobe visualization, detailed morphological and volumetric assessment, precise delineation of adjacent structures, and comprehensive cervical lymph node survey 6 15. Computed Tomography (CT) is less frequently used, mainly when MRI is contraindicated or in cases of claustrophobia 17.
This study aims to identify the most informative MRI features for the differential diagnosis of parotid tumors, distinguishing between benign and malignant entities.
Materials and Methods
This retrospective study analyzed pre-surgical MRI scans of 94 patients (43 females, 51 males; mean age 43.5 years, range 15–92 years) with parotid gland tumors who underwent surgical resection at the Department of Otolaryngology of Catholic University of the Sacred Heart between February 2005 and September 2014.
The MRI protocol consistently included T1-weighted (T1w), T2-weighted (T2w), and contrast-enhanced fat-saturated T1w sequences. Ethical approval was obtained, and all patients provided informed consent. Histopathological and immunohistochemical analyses of surgical specimens revealed 69 benign (73%) and 25 malignant (27%) tumors. The benign group comprised 44 pleomorphic adenomas, 18 Warthin’s tumors, and 7 other benign tumors (1 haemangioma, 2 myoepitheliomas, 1 ductal cyst, 1 lipoma, 1 oncocytoma, 1 cholesteatoma). The malignant group included 6 squamous cell carcinomas, 3 carcinoma ex pleomorphic adenomas, 2 mucoepidermoid carcinomas, 1 adenoid cystic carcinoma, and 13 other malignant tumors (1 sebaceous tumour, 1 myoepithelial carcinoma, 4 undifferentiated tumours, 5 metastases from squamous cell carcinomas of the skin and 2 adenocarcinomas).
Two radiologists with extensive experience in head and neck imaging (24 and 12 years respectively) independently reviewed all pre-surgical MRI scans, evaluating the following parameters crucial for Parotid Mass Differential Diagnosis:
- Location and Number of Lesions: Unilateral or bilateral, single or multifocal. Superficial lobe (body, anterior and inferior processes) or deep lobe location, using a line from the stylomastoid foramen to the retromandibular vein’s lateral margin to differentiate lobes.
- Overall Shape: Oval, round, lobulated, or irregular.
- Size: Maximum diameter.
- Margins: Well-defined, ill-defined, or spiculated, assessed on pre- and post-contrast T1w images.
- Extraglandular Growth: Invasion of adjacent tissues (subcutaneous fat, skin, masticatory space, mandible). In multifocal cases, infiltrative growth in at least one lesion classified the overall growth pattern as infiltrative.
- Capsule: Hypointense rim on T1w and T2w images, with or without contrast enhancement.
- Signal Intensity (SI) and Enhancement: T1w SI relative to masseter muscle (hypointense, isointense, hyperintense); T2w SI relative to normal parotid parenchyma (hypointense, isointense, hyperintense). Contrast enhancement classified as low, intermediate, or strong compared to normal parotid tissue, and homogeneity of enhancement (homogeneous or heterogeneous). For heterogeneous lesions, the predominant portion was assessed.
- Cystic Content SI: T1w and T2w SI of cystic areas relative to the lesion (hypointense, isointense, hyperintense).
- Perineural Spread: Pathological enhancement along cranial nerves (V and VII).
- Cervical Adenopathy: Rounded morphology, short axis > 1 cm, necrosis, lymph node conglomerates, extranodal extension.
In cases with multiple lesions, the largest lesion’s characteristics were analyzed. Fisher’s exact test was used for statistical comparison of MRI findings between malignant and benign tumors (Table 1). A p-value < 0.05 indicated statistical significance.
Table 1. Results of statistical analysis.
| Findings | Malignant Tumours (n = 25) | Benign Tumours (n = 69) | p Value, Fisher’s Exact Test | PPV¶ | NPV¶ | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | ||||
| Signal Intensity† | |||||||
| T1- T1+ T1= T1* T2- T2+ T2= | 3 15 7 0 2 20 3 | 22 10 18 25 23 5 22 | 9 35 25 13 1 63 5 | 60 34 44 56 68 6 64 | 1 0.4879 0.6229 0.0175 0.1716 0.1544 0.4346 | 0.25 0.3 0.22 0 0.67 0.24 0.38 | 0.73 0.77 0.71 0.69 0.75 0.55 0.74 |
| CE‡ | |||||||
| 0 1 2 3 | 0 2 5 18 | 25 23 20 7 | 3 7 24 35 | 66 62 45 34 | 0.5625 1 0.2118 0.0987 | 0 0.22 0.17 0.34 | 0.73 0.73 0.69 0.83 |
| Appearance | |||||||
| Homogeneous Inhomogeneous | 1 24 | 24 1 | 29 40 | 40 29 | 0.03 0.38 | 0.63 0.97 | 0.58 0.04 |
| Margins | |||||||
| Well-defined Ill-defined or spiculated | 3 22 | 22 3 | 66 3 | 3 66 | 0.04 0.88 | 0.12 0.96 | 0.88 0.96 |
| Morphology | |||||||
| Round Oval Lobulated Irregular | 1 11 9 4 | 24 14 16 21 | 2 39 28 0 | 67 30 41 69 | 1 0.3516 0.8124 0.0041 | 0.33 0.22 0.24 1 | 0.74 0.68 0.72 0.77 |
| Cists/necrosis | 10 | 15 | 21 | 48 | 0.458 | 0.32 | 0.76 |
| Sites | |||||||
| Body Lower extension Anterior extension Parapharyngeal extension More sites Superficial lobe Deep lobe Single lesion Multifocal | 13 2 1 5 4 10 15 20 5 | 12 23 24 20 21 15 10 5 20 | 24 17 14 7 7 47 22 65 4 | 45 52 55 62 62 22 47 4 65 | 0.1557 0.0885 0.0633 0.2919 0.4752 0.0020 0.0020 0.0530 0.0530 | 0.35 0.11 0.07 0.42 0.36 0.18 0.41 0.24 0.56 | 0.79 0.69 0.7 0.76 0.75 0.59 0.82 0.44 0.76 |
| Adenopathy | 12 | 1 | 13 | 68 | 0.92 | 0.84 | 0.99 |
| Perineural spread | 1 | 24 | 0 | 69 | 0.2660 | 1 | 0.74 |
| Infiltrative grown pattern | 17 | 8 | 0 | 69 | 1 | 0.9 | 1 |
†T1- hypointensity with respect to masseter muscle, T1+ hyperintensity with respect to masseter muscle, T1= isointensity with respect to masseter muscle, T1* cysts hyperintense on T1 with respect to lesion, T2- hypointensity with respect to parotid tissue, T2+ hyperintensity with respect to parotid tissue, T2= isointensity with respect to parotid tissue;
‡0 = no contrast enhancement, 1: low contrast enhancement, 2: intermediate contrast enhancement, 3: strong contrast enhancement;
¶PPV: positive predictive value, NPV: negative predictive value.
Results
Malignant tumors were more prevalent in males (M:F = 2.1:1), while benign tumors showed a near-equal gender distribution (M:F = 1.02:1), although this gender difference was not statistically significant for malignancy (p = 0.09). Among benign lesions, Warthin’s tumor was significantly more common in males (p = 0.012; M:F = 4.5:1), whereas pleomorphic adenomas exhibited a slight female preponderance (M:F = 1:1.4). The mean age at diagnosis for malignant tumors was 65.2 years (range 25–92 years) and 50.7 years for benign tumors (range 15–89 years), indicating a trend for malignancy in older patients (p < 0.001).
Multifocal lesions were observed in nine patients: five with skin metastases of squamous cell carcinoma, three with Warthin’s tumors, and one with pleomorphic adenoma. All multifocal lesions were unilateral except for the pleomorphic adenoma and one Warthin’s tumor case. Both benign (24/69) and malignant (13/25) lesions frequently localized to the parotid gland body. Warthin’s tumors showed a higher predilection for the inferior process (10/18).
Irregular shape was noted in 16% (4/25) of malignant tumors, while absent in benign lesions (p = 0.004; specificity 1, sensitivity 0.16). Conversely, round, oval, or lobulated shapes were observed in 100% of benign and 84% of malignant tumors (Table 1). Malignant tumors were, on average, larger (38 mm) than benign tumors (28 mm) (p = 0.012). A significant finding was that 88% of malignant tumors displayed ill-defined margins compared to only 0.04% of benign tumors (p < 0.001) (Fig. 1; Table 1). Extraglandular infiltrative growth was present in 17 of 25 malignant tumors (p < 0.001) and absent in benign cases.
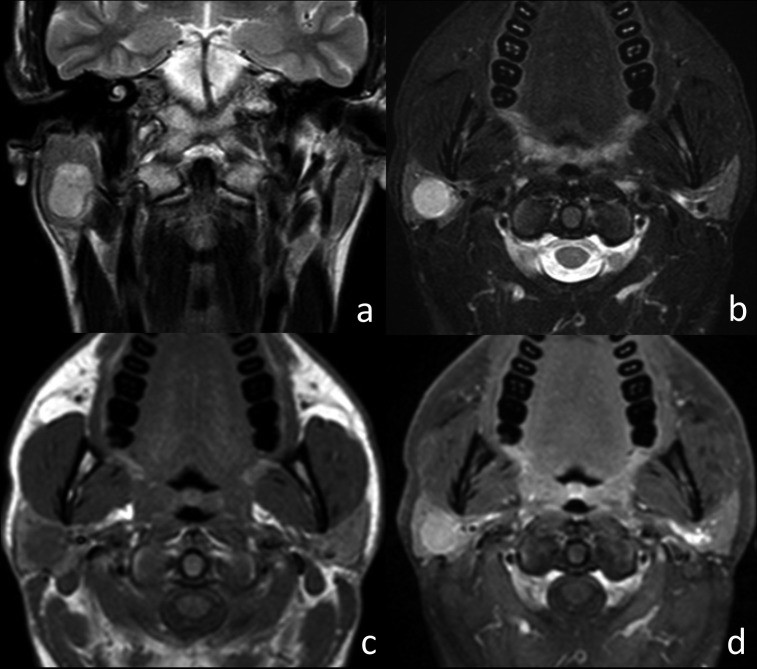
Fig. 1.
MRI characteristics of squamous carcinoma of the left parotid gland. Axial T2w image (a), axial T1w image (b), and axial T1w fat-sat image after contrast injection (c and d). The tumor (arrowheads) shows hypointensity on T2w and isointensity on T1w with ill-defined margins. Post-contrast (T1w fat-sat), strong enhancement and encasement of the external carotid artery (arrow) are evident. Cervical adenopathies are also present in levels IIa and IIb (thick arrows).
A hypointense capsule was more commonly associated with benign tumors (56% vs 8% in malignant tumors), indicating a predictive value for benignity (PPV 0.95, specificity 0.92, sensitivity 0.57, p < 0.001) (Table 1). Cystic or necrotic areas were not helpful in differentiating benign from malignant tumors, observed in 40% of malignant and 30% of benign lesions (p = 0.458). Cervical adenopathy was significantly more frequent in malignant tumors, observed in almost 50% (12/25) (p < 0.001). Perineural spread was rare, identified in only one malignant case and no benign cases (p = 0.266).
Pleomorphic adenomas and Warthin’s tumors, the most common benign entities, comprised approximately 90% of benign parotid tumors. Typical MRI features for pleomorphic adenomas included strong contrast enhancement (p < 0.001) (Fig. 2). For Warthin’s tumors, hyperintense cystic areas on T1w images were characteristic (p < 0.001) (Fig. 3).
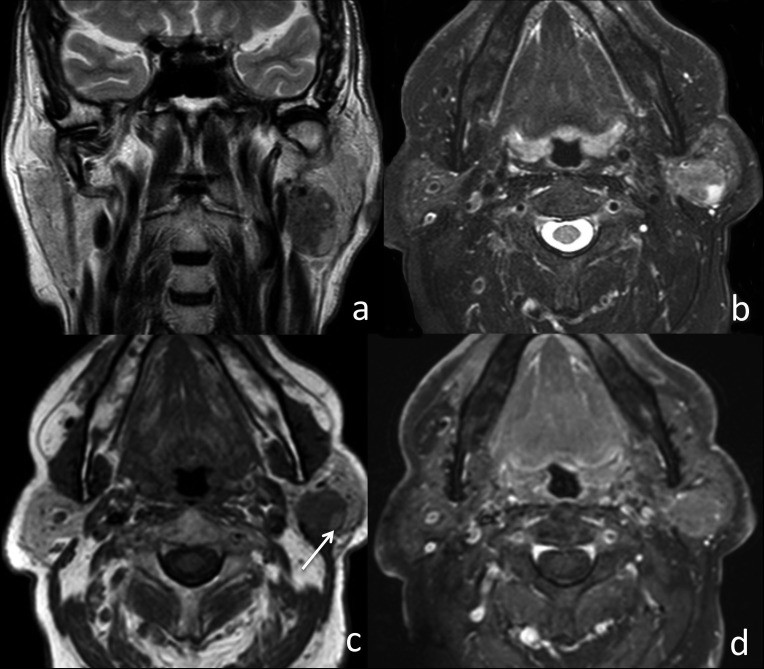
Fig. 2.
MRI features of pleomorphic adenoma in the right parotid gland. Coronal T2w image (a), axial T2w fat-sat image (b), axial T1w image (c), and axial T1w fat-sat image post-contrast (d). The oval lesion is hyperintense on T2w, isointense on T1w, with sharp margins and homogeneous signal intensity. Strong contrast enhancement, exceeding normal parotid tissue, is observed.
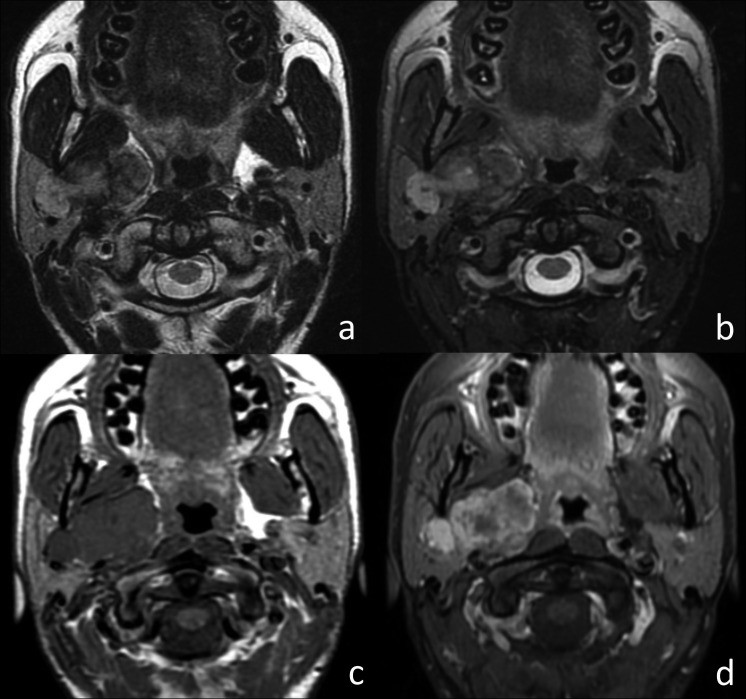
Fig. 3.
MRI characteristics of Warthin’s tumor in the left parotid gland. Coronal T2w image (a), axial T2w fat-sat image (b), axial T1w image (c), and axial T1w fat-sat image post-contrast (d). The oval lesion exhibits well-defined margins and hypointensity on T2w images. A hyperintense cystic area (arrow) is visible on T1w images. Focal contrast enhancement is noted post-contrast.
Other histological subtypes were limited in number, precluding statistically significant results for individual analyses.
Discussion
Existing literature consistently highlights ill-defined margins, inhomogeneous signal intensity (SI), and low T2w SI as typical MRI features of malignant parotid tumors, often accompanied by cervical adenopathy or perineural spread. Conversely, benign tumors are generally characterized by well-defined margins, high T2w SI, and superficial lobe location 6 17–19.
Our findings corroborate that ill-defined margins are the most valuable MRI feature in differentiating benign and malignant parotid lesions (p < 0.001). This aligns with previous studies emphasizing margin definition as a strong predictor of malignancy 17 18. However, ill-defined margins are not entirely specific for malignancy and can be seen in benign tumors with associated inflammation, as observed in two Warthin’s tumors and one haemangioma in our study, potentially leading to diagnostic ambiguity. Cystadenolymphomas are known to exhibit this feature, and inflammatory changes may arise from bacterial contamination or infarction of a Warthin’s tumor, triggering a foreign body reaction 20–22.
Conversely, malignant transformation can occur within well-defined lesions, particularly in pleomorphic adenomas, with reported rates up to 25% 6 17 23. We encountered three carcinoma ex pleomorphic adenoma cases with microscopic carcinomatous foci (two adenocarcinoma NOS and one mucoepidermoid carcinoma), which were challenging to detect on MRI but identified histologically. Pathological cervical adenopathy was another significant indicator of malignancy (p < 0.001), consistent with prior research 24. Extraglandular infiltrative growth into surrounding tissues also strongly suggested malignancy (p < 0.001) 19 20. Tumor size also showed correlation with malignancy, with malignant lesions being larger on average (p = 0.012).
Signal intensity of solid components was less discriminatory in distinguishing benign from malignant tumors (p = 0.154). Contrary to some studies, the majority of both malignant (80%) and benign (91.3%) tumors showed T2-weighted SI higher than normal parotid parenchyma. While hypointense T2w SI is highly specific for malignancy (0.99), its low sensitivity (0.08) and infrequent occurrence in our series limited its statistical significance (p = 0.17) 17 18.
Inhomogeneous SI also lacked specificity for malignancy, present in 96% of malignant and 58% of benign tumors, exhibiting a low specificity for malignancy (0.04). Inhomogeneous SI in larger benign lesions might be attributed to necrosis, hemorrhage, and cystic degeneration (Fig. 4) 6 17. Cystic or hemorrhagic areas, in general, were not useful in differentiating benign from malignant tumors. However, T1w hyperintense cysts within well-defined lesions strongly suggested Warthin’s tumor (p < 0.001) 19 25 26.
Fig. 4.
MRI appearance of an atypical pleomorphic adenoma of the right parotid gland. Axial T2w image (a), axial T2w fat-sat image (b), axial T1w image (c), and axial T1w fat-sat image post-contrast (d). The lobulated tumor, involving both parotid lobes, shows sharp margins and inhomogeneous SI on T2w images. Strong inhomogeneous enhancement is seen post-contrast.
Contrast enhancement improved margin definition and delineation of solid versus cystic components but did not significantly differentiate benign from malignant tumors based on enhancement degree alone. However, contrast enhancement patterns differed between pleomorphic adenomas and Warthin’s tumors, with pleomorphic adenomas showing strong or complete enhancement (p < 0.001) 18 27 28. Lesion location was not a reliable predictor of malignancy, with the parotid body being the most frequent site for both tumor types. Consistent with previous reports, the inferior process was the most common location for Warthin’s tumor (55%, p = 0.01) 17.
A limitation of this study is the lack of routine DWI and perfusion imaging, which could provide further insights into parotid lesion characterization 29–32. Furthermore, the predominance of benign lesions, particularly pleomorphic adenomas and Warthin’s tumors, and the heterogeneity of malignant subtypes limited the ability to define specific MRI patterns for each malignant entity.
Conclusions
MRI is the optimal imaging modality for parotid gland tumors. Careful evaluation of lesion margins, cystic content SI, extraglandular growth, cervical lymph nodes, and perineural spread can significantly aid in the differential diagnosis between benign and malignant parotid lesions. Ill-defined margins are a strong indicator of malignancy, while well-defined margins and a hypointense capsule are more characteristic of benign lesions.
References
[1] Eveson JW, Cawson RA. Tumours of the salivary glands: a review of 2,410 cases. Int J Oral Maxillofac Surg. 1985;14 Suppl 1:17-28.
[2] Auclair PL. Benign salivary gland tumors. In: Gnepp DR, ed. Diagnostic surgical pathology of the head and neck. Philadelphia: WB Saunders; 2001:977-1051.
[3] Zbären P, Tschudi D, Kappeler L, Stauffer E. Primary parotid carcinoma: prognostic factors and long-term results of surgery with or without postoperative radiotherapy. Head Neck. 2004;26(6):515-21.
[4] Roh JL, Park CI. Prognostic factors of surgically resected parotid gland carcinoma: retrospective analysis of 170 cases. Laryngoscope. 2008;118(11 Pt 1):1955-62.
[5] Bradley PJ. Malignant tumours of the salivary glands: a clinicopathological review. J Maxillofac Surg. 1983;11(2):58-67.
[6] Teymoortash A, Werner JA. Salivary gland tumors: diagnostics, therapy and prognosis. Dtsch Arztebl Int. 2008;105(42):677-82.
[7] O’Brien CJ, Soong SJ, Herrera GA, Urist MM, Balch CM. Malignant salivary gland neoplasms. Analysis of prognostic factors and survival. Am J Surg. 1986;152(6):610-6.
[8] Schmidt RL, Halling AC, Wampfler JA, Lingle WL, Weaver AL, 확인용: 데이타 없음, et al. A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland neoplasms. Am J Clin Pathol. 2011;136(1):45-59.
[9] Heller KS, Dubner S, Chess Q. Needle tract seeding after fine-needle aspiration of salivary gland tumors. Arch Otolaryngol Head Neck Surg. 1992;118(12):1335-7.
[10] Cohen MB, Fisher PE, Holly EA, Ahn DK, Miller TR. Fine needle aspiration cytology diagnosis of salivary gland lesions. Acta Cytol. 1984;28(3):235-9.
[11] Young JA. Diagnostic problems in salivary cytology. Adv Anat Pathol. 2003;10(5):284-97.
[12] McGurk M, Thomas GJ. Radical versus conservative surgery in the management of benign parotid tumours. Br J Surg. 2003;90(11):1329-30.
[13] Roh JL. Extracapsular dissection versus superficial parotidectomy for primary benign parotid tumors: a systematic review and meta-analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;112(2):162-70.
[14] Choi JS, Lee JH, Cha IH, Kim MS, Lee SS, Choi KH. Diagnostic accuracy of ultrasound in the preoperative evaluation of parotid gland tumors. J Ultrasound Med. 2013;32(11):1955-63.
[15] Gondim DD, Gondim GC, Filho JG, Brasileiro IC. Magnetic resonance imaging of parotid gland tumors: pictorial essay. Radiol Bras. 2014;47(2):111-6.
[16] Terraz S, Becker M. Imaging of salivary gland tumours. Cancer Imaging. 2011;11 Spec No A:S3-11.
[17] Williams MD. Imaging of salivary glands. Semin Ultrasound CT MR. 2013;34(2):119-30.
[18] Izumi M, Ishida Y, Kasai M, Moriyama H, Shibuya H. MR imaging of parotid tumors: correlation with histologic diagnosis. AJR Am J Roentgenol. 1997;169(5):1421-6.
[19] Castro-Villegas MC, Lopez-Rios F, Burgos JS, Perez-Campos A, Alvarez-Santullano V, Ciria-Recuenco F. MR imaging of parotid gland tumours: correlation with histopathologic diagnosis. Eur Radiol. 2001;11(8):1422-9.
[20] Seifert G, Langrock I, Löning T. Warthin’s tumor. Cystadenolymphoma. In: Seifert G, Sobin LH, eds. Histological typing of salivary gland tumours. Berlin: Springer-Verlag; 1991:62-6.
[21] Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumours. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005.
[22] Bajaj Y, Singh S, Gupta R, Bhatnagar A, Mannan R, Thompson L. Inflammatory change in Warthin’s tumour: a diagnostic dilemma. J Laryngol Otol. 2010;124(8):894-7.
[23] Luna MA, Batsakis JG, Guillamondegui OM. Carcinomas ex pleomorphic adenoma of the parotid gland. Cancer. 1987;59(9):1767-71.
[24] Lydiatt DD, Patel SG, O’Sullivan B, Brandwein-Gensler M, Ferris RL, Ganly PS, et al. Head and neck cancers–major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(2):93-103.
[25] Vogl TJ, Brüning R, Schedel H, Grevers G, Hahn D. Parotid gland tumors: value of MR imaging in differentiation between benign and malignant lesions. AJR Am J Roentgenol. 1991;157(3):573-7.
[26] Suei Y, Taguchi A, Tanimoto K, Wada T. MRI features in differential diagnosis of pleomorphic adenoma and Warthin’s tumor of the parotid gland. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97(2):249-56.
[27] Jensen JL, Barnes L, Nelson JF Jr. Cystadenolymphoma (Warthin’s tumor): a clinicopathologic study of 125 cases. Cancer. 1976;37(2):846-9.
[28] Seifert G, Donath K, Engelhardt W, Weber R. Multicentricity of cystadenolymphoma (Warthin’s tumor). Diagnostic criteria and differential diagnosis. Virchows Arch A Pathol Anat Histol. 1978;377(3):185-99.
[29] Motosugi U, Bettschart V, Delattre BM, Becker M. Parotid gland tumours: diagnostic value of diffusion-weighted MR imaging. Eur Radiol. 2009;19(4):919-26.
[30] Takebayashi S, Hosokawa T, Miyata T, Taguchi K, Fujiwara T, Yamashita Y. Diffusion-weighted MR imaging of parotid gland tumors: is ADC useful for differentiating benign from malignant tumors? AJR Am J Roentgenol. 2009;193(4):W306-11.
[31] Nakanishi K, Sako S, Kishimoto H, Hosoi H, Nakamoto M, Baba Y, et al. Usefulness of apparent diffusion coefficient value and signal intensity ratio in diffusion-weighted imaging for differential diagnosis of parotid gland tumors. J Magn Reson Imaging. 2011;34(3):613-9.
[32] Herzog M, Gödde D, Kinner S, Weber F, Böning G, Arndt C, et al. Dynamic contrast-enhanced perfusion MRI for differentiation of benign and malignant parotid gland tumors. J Magn Reson Imaging. 2011;33(1):126-32.