Introduction
Peritoneal carcinomatosis (PC), the widespread dissemination of cancer within the peritoneal cavity, is a frequent and severe complication of numerous malignancies, most notably those originating from the colorectal, gastric, and gynecological systems. Diagnosing PC presents a significant clinical challenge due to its varied origins and the broad spectrum of conditions that can mimic its presentation. These differential diagnoses range from primary peritoneal malignancies to benign conditions such as endometriosis and inflammatory diseases. The insidious nature of PC often leads to late diagnosis, underscoring the critical role of effective and timely diagnostic strategies. This article provides an in-depth review of Peritoneal Carcinomatosis Diagnosis, emphasizing the pivotal role of imaging modalities in detection, staging, and treatment planning. We will explore the pathophysiology, typical imaging findings, the utility of different imaging techniques including ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography/computed tomography (PET/CT), and discuss the Peritoneal Carcinomatosis Index (PCI) as a crucial tool in assessing disease burden and guiding surgical decisions. A thorough understanding of these aspects is paramount for radiologists and clinicians to improve diagnostic accuracy and ultimately enhance patient outcomes in the face of this challenging condition.
Peritoneal Anatomy, Physiology, and Metastatic Pathways
The Peritoneal Cavity and Fluid Dynamics
The peritoneal cavity is a complex anatomical space situated between the parietal and visceral peritoneum. The parietal peritoneum lines the abdominal wall and diaphragm, actively secreting peritoneal fluid, while the visceral peritoneum envelops the abdominal organs, providing them with mobility and facilitating fluid transport. This cavity, in a healthy state, contains a minimal volume of serous fluid, typically 5-20ml, composed primarily of plasma transudate and ovarian exudate. This fluid acts as a lubricant, enabling frictionless organ movement and mediating the exchange of substances and immune cells within the peritoneal environment.
The circulation pattern of peritoneal fluid plays a crucial role in the dissemination of peritoneal metastases. Fluid within the pelvic recesses tends to ascend towards the subphrenic space, predominantly through the right paracolic gutter, a deeper channel that offers less resistance. The upward flow on the left side is partially impeded by the phrenicocolic ligament. Additionally, the falciform ligament acts as a barrier, preventing fluid mixing between the right and left subphrenic spaces. However, in cases of significant ascites accumulation, these anatomical barriers can be overcome, leading to a more widespread distribution of fluid and, consequently, metastatic deposits. Understanding these fluid dynamics is essential for predicting and interpreting the distribution patterns of peritoneal carcinomatosis on imaging.
Common Origins of Peritoneal Metastases
Peritoneal carcinomatosis represents the most frequent form of diffuse peritoneal malignancy. While it can arise from various primary cancers, ovarian and gastrointestinal carcinomas exhibit the highest propensity for peritoneal dissemination. Specifically, colorectal and gastric cancers are leading sources, followed by gynecological malignancies, particularly ovarian cancer. Other significant primary sites contributing to PC include cancers of the pancreas, breast (notably the most common extra-abdominal origin), appendix, biliary tract, liver, lung, and genitourinary tract. Recognizing the common primary origins is crucial for clinicians as it influences diagnostic approaches, treatment strategies, and prognostic considerations in patients suspected of having peritoneal carcinomatosis.
Imaging Findings in Peritoneal Carcinomatosis Diagnosis
The diagnostic imaging of peritoneal carcinomatosis relies on identifying characteristic features that indicate peritoneal involvement. The hallmark imaging findings include ascites, infiltration of the omentum and mesentery, and the presence of peritoneal nodules. In some instances, complications such as bowel or ureteral obstruction may also be observed, further supporting the diagnosis.
Ascites, the abnormal accumulation of fluid within the peritoneal cavity, is present in approximately 70% of patients with peritoneal carcinomatosis. This fluid build-up is attributed to two primary mechanisms: impaired reabsorption of peritoneal fluid at the level of the right subdiaphragmatic lymphatic chain and increased fluid secretion directly into the peritoneum due to vascular permeability factor (VPF) secreted by tumor cells. Imaging can readily demonstrate ascites, providing an important clue towards the diagnosis of PC.
Omental and mesenteric infiltration manifests as increased density and nodular changes within the omental fat, often described as “omental caking”. Further imaging signs include solid, stellate masses within the mesentery, clustering of small bowel loops, thickening of the gastric wall, scalloping of the liver surface, and peritoneal thickening, which can appear smooth, nodular, or plaque-like. Abnormal enhancement of the peritoneum following contrast administration is also a significant indicator. A distinctive “sleeve-like” pattern of tumor growth along the bowel serosa may also be observed.
Peritoneal nodules, representing direct tumor implants, are typically found in specific locations influenced by peritoneal fluid flow and anatomical recesses. These preferential sites include the paracolic gutters, the pouch of Douglas, the sigmoid mesocolon, the ileocecal region, and the parietal peritoneum along the anterior abdominal wall. While less common, calcification or cystic components may be present within these nodules.
It’s important to note that computed tomography (CT) can yield false-negative results, particularly in cases involving numerous, small peritoneal micronodules. The presence of ascites, however, improves the detection rate of parietal peritoneal nodules. Optimal visualization of visceral peritoneal and mesenteric involvement requires proper opacification of the bowel lumen and sufficient intraperitoneal fat.
Table 1: Common Sites of Peritoneal Implants
| Anatomical Location | Specific Sites |
|---|---|
| Hepatic Region | Hepatic ligaments, Gallbladder fossa, Splenic and hepatic surface, Morrison pouch, Gastrohepatic ligament, Hepatic porta |
| Mesentery and Omentum | Transverse and sigmoid mesocolon, Paracolic gutters, Small bowel mesentery, Greater omentum |
| Bowel Serosa | Small bowel serosa |
| Pelvic Region | Pouch of Douglas and uterovesical recess (females), Rectovesical recess (males) |
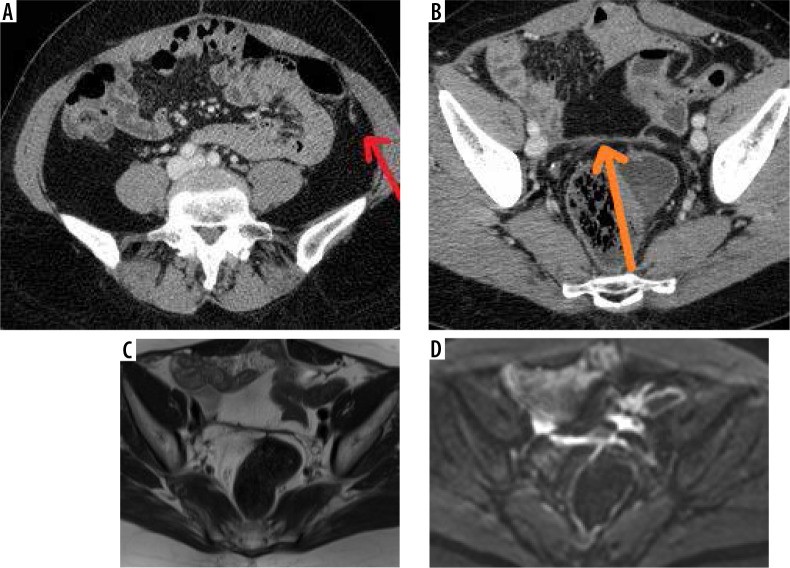
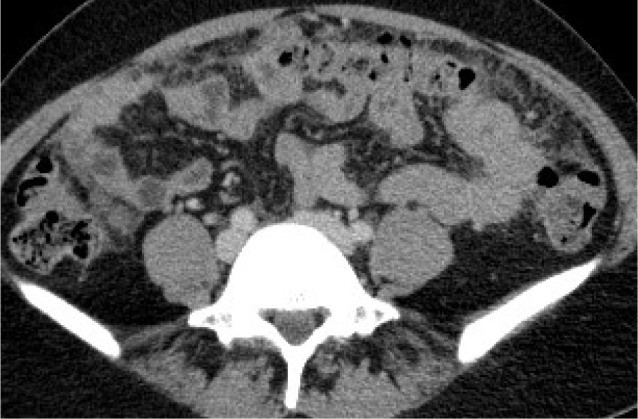

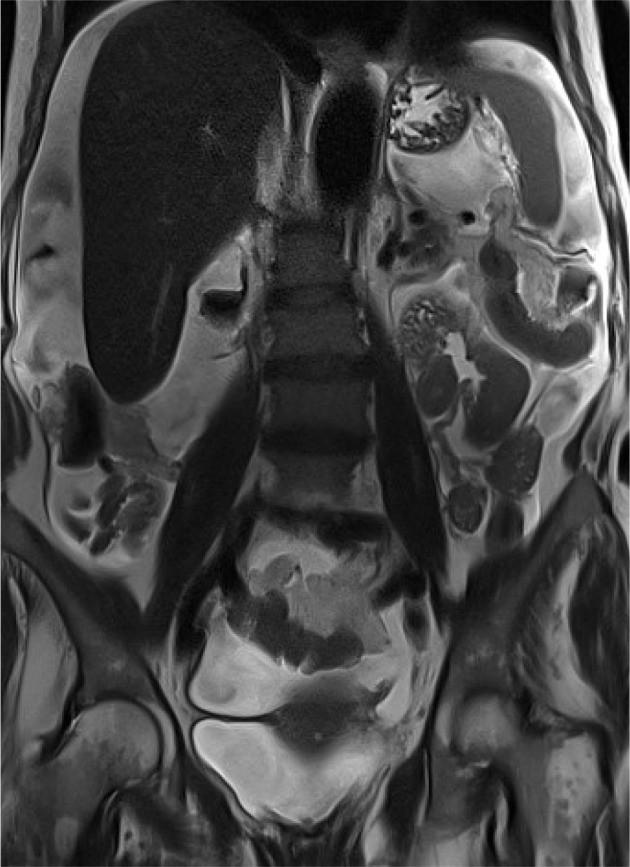
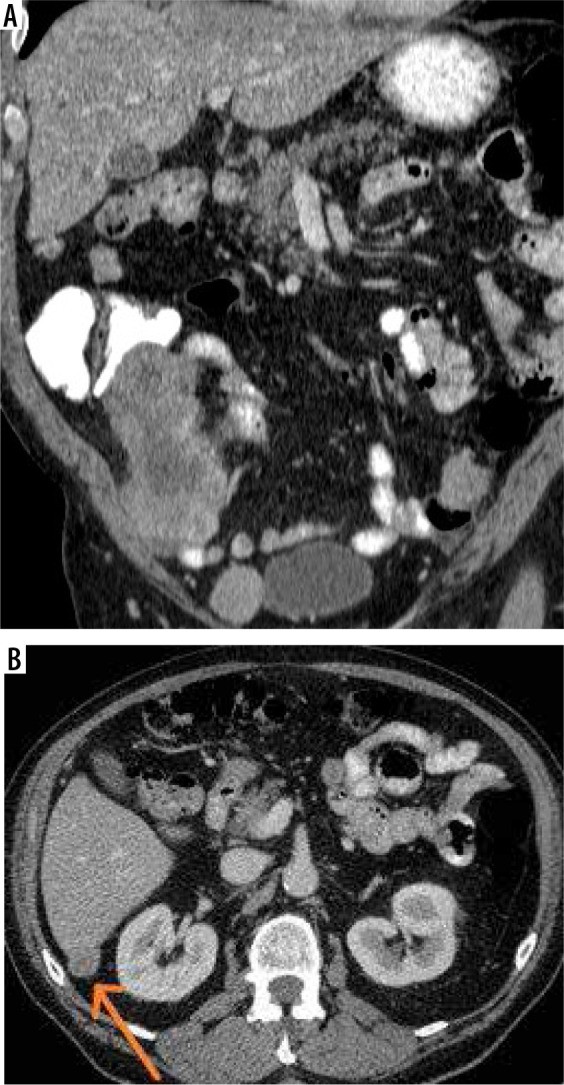
The Crucial Role of Diagnostic Imaging in Peritoneal Carcinomatosis
Diagnostic imaging plays a pivotal role in the management of peritoneal carcinomatosis. Its primary function is to differentiate between patients who are suitable candidates for cytoreductive surgery (CRS) and those with extensive or unfavorably located disease who would benefit more from neoadjuvant or palliative chemotherapy. This selection process is critical because CRS, while potentially life-prolonging, is associated with significant morbidity (23-35%) and mortality (2-4%).
Preoperative imaging not only guides treatment strategy but also aids in identifying suspicious areas that should be targeted for biopsy, in addition to standard sampling of the omentum, diaphragm, mesentery, and retroperitoneal lymph nodes. Furthermore, imaging findings, in conjunction with serum CA-125 levels, are used to monitor treatment response and detect disease recurrence. Therefore, accurate and comprehensive imaging is indispensable throughout the clinical course of peritoneal carcinomatosis.
Imaging Modalities for Peritoneal Carcinomatosis Diagnosis
Ultrasound (US)
Ultrasound is frequently employed as an initial imaging modality, particularly in patients presenting with abdominal distension. However, it has limited accuracy for staging peritoneal carcinomatosis. Ultrasound demonstrates a sensitivity of only 69% for detecting peritoneal metastases, 32% for nodal lesions, and 57% for parenchymal metastases. Despite these limitations, US can be valuable for guiding biopsies in patients requiring histological confirmation prior to chemotherapy.
On ultrasound, peritoneal implants typically appear as hypoechoic to isoechoic nodular or plaque-like lesions, often with color Doppler flow, or as diffuse peritoneal thickening. A combination of transabdominal and transvaginal approaches optimizes the diagnostic yield. The presence of bowel gas can hinder the detection of bowel wall and mesenteric implants, while ascites paradoxically improves visualization.
Interestingly, some studies suggest that in specific anatomical regions, ultrasound may exhibit better performance than CT. For example, a Chinese study indicated ultrasound superiority over CT in detecting pelvic metastases, and splenic and hepatic subcapsular metastases. Ultrasound also demonstrated higher sensitivity in detecting bowel serosal metastases compared to CT in this study. Conversely, CT proved more effective in identifying mesenteric, lateral peritoneal, and omental implants. A Czech study in ovarian cancer patients demonstrated that combined transvaginal and transabdominal ultrasound had good accuracy in predicting rectosigmoid infiltration, with high specificity but low sensitivity. Therefore, while not the primary modality for staging, ultrasound can play a complementary role in specific clinical scenarios and anatomical locations.
Computed Tomography (CT)
Computed tomography (CT) is considered the foundational imaging modality for staging and follow-up of peritoneal carcinomatosis. While exposure to ionizing radiation is a consideration, it is generally outweighed by the diagnostic benefits in oncologic patients. Modern CT technology achieves a sensitivity of 79-86% and specificity of 82-89% in detecting peritoneal involvement. However, the sensitivity significantly decreases to 7-28% when lesions are smaller than 10 mm.
CT sensitivity is also influenced by the anatomical location of implants, particularly in the absence of ascites. Metastases in the lesser omentum, mesenteric root, left subdiaphragmatic compartment, and small bowel serosa are frequently missed. The administration of positive oral contrast may enhance the detection of bowel wall implants but can negatively impact the visualization of calcified metastases. Factors such as low tissue contrast, peristalsis artifacts, inadequate bowel distention, postoperative anatomical distortion, and small implant size can further compromise CT’s diagnostic accuracy. A major advantage of CT remains its ability to simultaneously assess for pulmonary metastases and lymphadenopathy.
Optimal CT protocols for PC involve meticulous technique. Proper contrast enhancement is crucial, achieved with approximately 600 mg of iodine per kg of lean body weight. Some centers advocate for the routine use of intravenous hyoscine butyl bromide to reduce bowel motion artifacts. Image acquisition typically includes arterial phase (35 seconds post-contrast) and venous phase (70 seconds post-contrast) imaging. Delayed-phase images (8-10 minutes) are obtained if ureteral obstruction is suspected.
Magnetic Resonance Imaging (MRI)
Magnetic resonance imaging (MRI), while generally more expensive and time-consuming than CT, is a valuable second-line modality for peritoneal carcinomatosis diagnosis. Contrast-enhanced MRI and CT exhibit comparable overall sensitivities (90-95.5%). However, MRI can surpass CT in detecting small, sub-centimeter lesions, particularly in the subphrenic region and bowel serosa. Both modalities show similar accuracy in relapse detection, although differentiating between benign and malignant lymphadenopathy remains a challenge for both.
Standard MRI protocols for PC include T1-weighted and T2-weighted images in axial and coronal planes, fat-suppressed T1W and T2W sequences, dynamic contrast-enhanced sequences, and diffusion-weighted imaging (DWI). Abnormal contrast enhancement of the peritoneum at 5 minutes post-contrast can be an early indicator of involvement. Some centers utilize oral contrast agents (water or pineapple juice) and intramuscular hyoscine butyl bromide to improve image quality.
The incorporation of DWI into standard MRI protocols for pelvic and abdominal imaging in ovarian cancer patients has been shown to increase lesion detection rates by 21-29%. DWI is particularly useful for staging, differential diagnosis, and treatment response evaluation. MRI with DWI can detect metastases larger than 5 mm.
Motion artifacts from respiration and cardiac activity can hinder the visualization of subdiaphragmatic and hepatic subcapsular lesions. The inherently high DWI signal from normal spleen, lymph nodes, and bowel mucosa can lead to false-positive findings. Conversely, false-negative results can occur due to increased ADC values in cystic, necrotic, and mucinous metastases. Another limitation of MRI is the need for specialized expertise in interpretation. MRI demonstrates good correlation with surgical findings in the pelvis and central abdomen, but its sensitivity for detecting bowel wall involvement is lower.
Emerging research suggests a correlation between apparent diffusion coefficient (ADC) values of peritoneal lesions and treatment response. Successful chemotherapy may lead to increased ADC values even before a reduction in lesion size. Furthermore, high pre-treatment ADC values may be associated with poor chemotherapy response. Dynamic contrast-enhanced MRI sequences are also being investigated for their ability to differentiate benign from malignant lesions and assess treatment response.
Nuclear Medicine: PET/CT and PET/MR
Positron emission tomography/computed tomography (PET/CT), primarily using the glucose analog 18F-FDG, offers metabolic information in addition to anatomical detail. PET/CT is based on the principle that cells with high metabolic activity, such as neoplasms and inflammatory lesions, exhibit increased glucose uptake. Peritoneal implants manifest as focal or diffuse areas of abnormal 18F-FDG uptake in the bowel serosa, omental fat, and peritoneum. PET/CT sensitivity ranges from 78 to 97%, with specificity around 55-90%.
False-negative PET/CT results can occur due to small, scattered serosal implants mimicking normal bowel activity, low metabolic activity in mucinous lesions, implant size below the method’s spatial resolution, and absent uptake in small pulmonary metastases. Conversely, PET/CT may detect nodal metastases prior to the development of lymphadenopathy.
PET/CT demonstrates high sensitivity (72-100%) and specificity (40-90%) in detecting clinically and biochemically silent relapse. While some studies have shown that adding PET/CT to routine imaging alters staging in 55-64% of cases and influences clinical decisions in 34-59%, the overall evidence regarding the added value of PET/CT before CRS compared to standard imaging remains debated.
PET/MR combines the metabolic insights of PET with the superior soft tissue contrast of MRI. Although data are currently limited, PET/MR is believed to be more useful in assessing local disease extent (e.g., in cervical and endometrial cancer) than in detecting peritoneal metastases specifically.
Choosing the Optimal Imaging Modality
Comparative studies have explored the effectiveness of different imaging modalities in peritoneal carcinomatosis diagnosis. One study involving a small cohort of 15 patients found MRI, PET/CT, and CT to be equally effective in detecting peritoneal dissemination, although the results were not statistically significant. MRI showed the highest sensitivity, while PET/CT had the highest specificity in this study. Conversely, a study by Low et al. demonstrated significantly superior performance of MRI compared to CT, with higher per-site sensitivity and specificity. Another study suggested that combining MRI and CT improves the accuracy of preoperative Peritoneal Carcinomatosis Index (PCI) assessment compared to CT alone, particularly in the central abdomen, pelvis, and left upper abdomen.
A meta-analysis by Laghi et al. recommended CT as the first-line imaging modality, acknowledging that it may underestimate preoperative PCI in a significant proportion of cases. MRI demonstrated higher sensitivity and specificity in this meta-analysis. When comparing CT and PET/CT, PET/CT showed higher sensitivity and specificity. Another study found that CT failed to accurately diagnose the extent of peritoneal carcinomatosis in 17.5% of cases. In patients with lower PCI scores, CT overestimated the disease burden in some and underestimated it in others. CT predicted optimal cytoreduction with a sensitivity comparable to laparoscopy, and combining both methods increased sensitivity further. Specificities were similar across these methods.
In the context of relapse detection, miliary nodules may be initially missed on imaging unless clinical and laboratory findings (e.g., rising CA-125 levels) prompt careful review. PET/CT should be considered in ovarian cancer survivors with rising CA-125 and equivocal imaging to detect nodal or extra-abdominal metastases, excluding the early postoperative period where findings may be misleading. Imaging findings can sometimes be discordant with serum marker levels, with extensive intraperitoneal recurrence occurring despite normal marker levels. Some experts recommend baseline MRI soon after surgery to differentiate normal postoperative changes from early recurrence, as postoperative changes typically resolve over time while relapse-related findings progress.
Differential Diagnoses of Peritoneal Carcinomatosis
Distinguishing peritoneal carcinomatosis from other conditions causing diffuse peritoneal involvement is crucial, as many differential diagnoses require medical rather than surgical management. Conditions that can mimic PC on imaging are listed in Table 2. Practically, lymphoma, tuberculosis, mesothelioma, and pseudomyxoma peritonei are among the primary differential diagnoses to consider.
Table 2: Differential Diagnoses of Peritoneal Carcinomatosis
| Category | Specific Conditions |
|---|---|
| Primary Peritoneal Malignancies | Malignant mesothelioma, Primary peritoneal carcinoma, Desmoplastic small round cell tumor (DSRCT), Primary peritoneal lymphoma |
| Benign Neoplasms & Tumor-like Conditions | Desmoid tumor, Solitary fibrous tumor (SFT), Pseudomyxoma peritonei, Peritoneal lymphomatosis, Carcinoid tumor |
| Non-Malignant Conditions | Peritoneal tuberculosis, Actinomycosis, Splenosis implants, Endometriosis, Foreign body reaction |
Treatment Approaches for Peritoneal Carcinomatosis
Currently, the combination of cytoreductive surgery (CRS) and intraperitoneal chemotherapy (HIPEC or EPIC) is considered the optimal treatment strategy for peritoneal carcinomatosis. The introduction of these techniques has significantly improved patient survival. Depending on the primary malignancy type, median survival has increased from 3-24 months to 40-62 months. Numerous studies highlight the benefits of CRS and HIPEC in patients with colorectal carcinoma, ovarian cancer, primary peritoneal carcinoma, neuroendocrine tumors, peritoneal mesothelioma, and gastric cancer.
However, these extensive procedures are associated with significant complications, including fistula formation, obstruction, and anastomotic leaks. Mortality rates are approximately 2-4%, with major morbidity ranging from 23-35%. Therefore, patient selection is critical, focusing on individuals in good general condition where optimal cytoreduction is achievable.
Cytoreductive Surgery (CRS)
Cytoreductive surgery (CRS), pioneered by Paul Sugarbaker, aims to remove all visible peritoneal deposits through extensive surgical procedures, including multiple organ resections and peritonectomy procedures. CRS encompasses procedures such as pelvic peritonectomy, greater omentectomy, lesser omentectomy, cholecystectomy, bowel resections, diaphragmatic stripping, and removal of the liver capsule, sigmoid, rectum, mesorectum, and female genital tract. Splenectomy is performed if splenic involvement is present.
Intraperitoneal Chemotherapy: HIPEC and EPIC
Hyperthermic intraperitoneal chemotherapy (HIPEC) is performed immediately following CRS in the operating room. Chemotherapeutic agents, heated to 41-43°C, are instilled into the peritoneal cavity, often with concurrent intravenous chemotherapy. Gentle patient movement ensures even distribution of the heated chemotherapy solution. After 1-1.5 hours, the fluid is removed, and the surgical incision is closed. HIPEC’s effectiveness is attributed to several mechanisms: hyperthermia enhances chemotherapy cytotoxicity and preferentially damages tumor cells; direct intraperitoneal drug delivery achieves higher local drug concentrations; and portal venous drainage of the HIPEC fluid delivers high chemotherapy concentrations to the liver, potentially eradicating hepatic micrometastases. Optimal cytoreduction (R0 and R1 resections) is crucial as chemotherapy penetration is limited.
EPIC (early postoperative intraperitoneal chemotherapy) involves administering several cycles of chemotherapy directly into the peritoneal cavity through percutaneous catheters in the early postoperative period. BIPSC/NIPS (bidirectional/neoadjuvant intraperitoneal and systemic chemotherapy) is a novel approach developed in Japan for gastric cancer, combining neoadjuvant systemic and intraperitoneal chemotherapy followed by CRS, HIPEC, and EPIC, aiming to reduce tumor burden, remove macroscopic disease, and eradicate microscopic residual disease.
Patient Eligibility for Surgery
Preoperative assessment of patient eligibility for CRS and HIPEC is paramount. Three main factors are considered: the extent of peritoneal involvement, the feasibility of complete cytoreduction, and the anticipated postoperative quality of life. Universal inoperability criteria are lacking, and decisions are often based on institutional experience. The radiologist’s role is to identify findings that may pose surgical challenges rather than simply labeling disease as resectable or unresectable.
Excluding distant metastases, pleural involvement, and retroperitoneal lymphadenopathy is essential, typically achieved with contrast-enhanced CT of the thorax, abdomen, and pelvis. MRI, ultrasound, and PET/CT may be used in complex cases. Differentiating intraparenchymal liver metastases from subcapsular implants is crucial, as intraparenchymal metastases are generally a contraindication to surgery, except in select colorectal cancer cases. Each lesion should be meticulously reported, including size, location, and relationship to surrounding structures. Assessment of small bowel involvement (focal vs. diffuse) and signs of ileus is critical. Large bowel serosal metastases or those near the bowel wall are negative predictive factors for obstruction. Enlarged lymph nodes and hernias should also be assessed for tumor involvement.
Laparotomy remains the gold standard for staging peritoneal carcinomatosis. Exploratory laparoscopy may be used in ambiguous cases and can reduce unnecessary CRS attempts significantly.
Contraindications to Cytoreductive Surgery
Table 3: Contraindications to Cytoreductive Surgery
| Absolute Contraindications | Relative Contraindications |
|---|---|
| High PCI score | High BMI |
| Incurable second malignancy | Acute ileus |
| Unresectable distant metastases | Acute infection |
| Extensive retroperitoneal involvement | Cardiac insufficiency |
| Poor general condition | Hepatic insufficiency |
| Hepatic hilum involvement | Renal insufficiency |
| Mesenteric root involvement | |
| Bladder trigone involvement | |
| Massive small intestine involvement | |
| Lesions penetrating the diaphragm |
PCI – Peritoneal Carcinomatosis Index, BMI – body mass index
The surgical approach may vary based on the primary cancer origin. Primary peritoneal neoplasms are often considered resectable even with stomach and colon involvement, unlike ovarian and colorectal carcinomatosis. Relative contraindications include high BMI, acute ileus, infection, and cardiac, hepatic, or renal insufficiency. Despite thorough preoperative radiological assessment, a significant proportion of patients are found to be inoperable at surgical exploration, often due to diffuse digestive tract involvement, mesenteric root invasion, and miliary peritoneal dissemination.
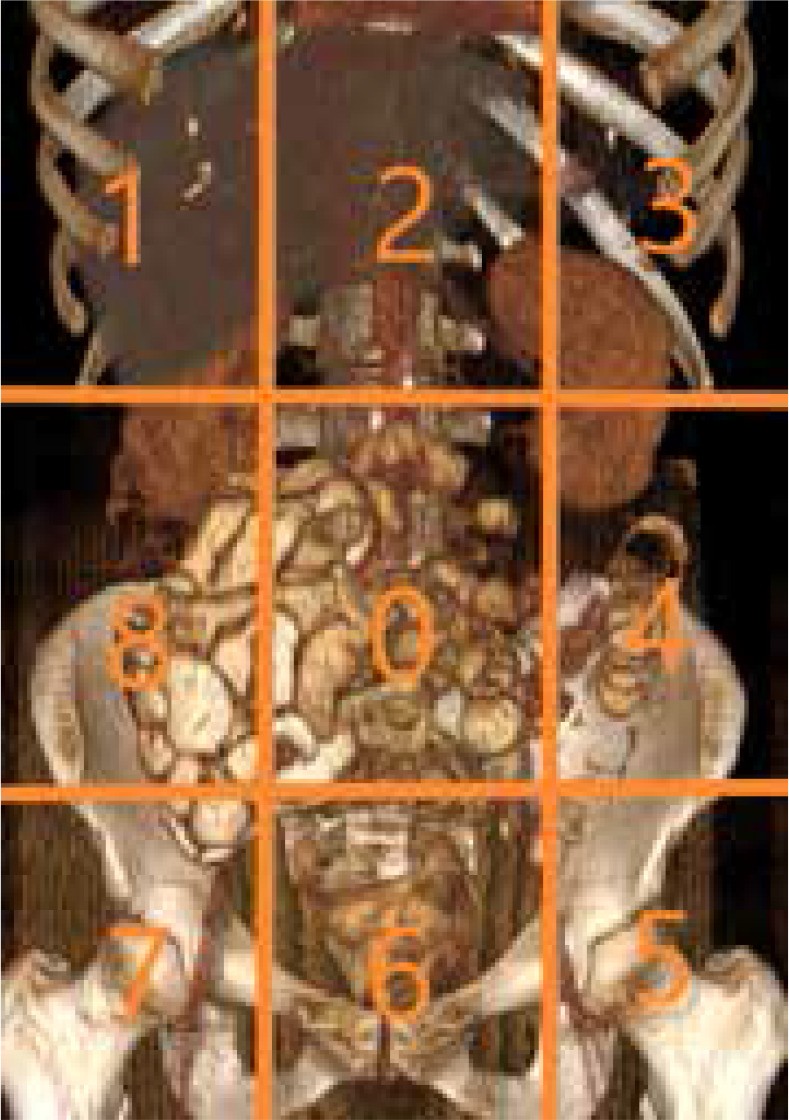
Peritoneal Carcinomatosis Index (PCI) and its Significance
The Peritoneal Carcinomatosis Index (PCI) is a scoring system initially developed for surgical assessment of tumor burden but is now also applied preoperatively using diagnostic imaging. The PCI divides the abdomen into 13 regions. Each region is examined, and lesion size is scored from 0 to 3 (0 = no tumor, 1 = metastases < 0.5 cm, 2 = metastases 0.5-5 cm, 3 = metastases > 5 cm or confluent). The PCI score is the sum of scores from all 13 regions, ranging from 0 to 39. The PCI score is a critical factor in determining surgical feasibility and prognosis. Various studies have proposed different PCI cut-off points for surgical candidacy.
Conclusion
Preoperative diagnostic imaging is indispensable in determining patient suitability for cytoreductive surgery and intraperitoneal chemotherapy in peritoneal carcinomatosis. A detailed radiology report accurately depicting crucial findings that may preclude CRS is essential to prevent unnecessary surgical interventions and associated morbidity and mortality. For patients deemed unsuitable for CRS and HIPEC, alternative treatments such as neoadjuvant or palliative chemotherapy can be considered. Continuous advancements in imaging techniques and interpretation are vital to improve diagnostic accuracy and ultimately optimize patient management in this complex oncologic condition.
References
References (reuse references from original article – ensure they are correctly formatted in markdown if needed, or list them as in the original).
[1] Sugarbaker PH. Peritoneal Carcinomatosis: Principles of Management. New York, NY: Springer; 2011.
[2] Esquivel J, Sticca RP, Sugarbaker PH. Peritoneal carcinomatosis and its treatment. World J Surg. 2002;26(3):313-320.
[3] Flessner MF. Peritoneal transport physiology. In: Ronco C, Bellomo R, Kellum JA, editors. Critical Care Nephrology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2009. pp. 1351–1358.
[4] Gore RM, Miller FH, Pickhardt PJ, et al. Peritoneal and mesenteric disease: radiologic-pathologic correlation. Radiographics. 2001;21(5):1251-1281.
[5] Ros PR, Bidlingmaier M, Weirich G, et al. Peritoneal and retroperitoneal metastases. Semin Ultrasound CT MR. 1995;16(5):341-355.
[6] Low RN, Barone RM. Peritoneal carcinomatosis: CT and MRI findings and diagnostic pitfalls. Abdom Imaging. 2010;35(6):643-657.
[7] Rubesin SE, Levine MS. Inflammatory diseases of the peritoneum, mesentery, and omentum. Radiol Clin North Am. 1996;34(4):715-735.
[8] Krestin GP, Steinbrich W. Peritoneal carcinomatosis: imaging diagnosis and staging. Eur Radiol. 2000;10(1):1-13.
[9] Jacquet P, Sugarbaker PH. Peritoneal carcinomatosis. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2899–2914.
[10] Van Ruth S, Verwaal VJ, Zoetmulder FA. Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Br J Surg. 2006;93(10):1181-1191.
[11] Jaeck D, Goéré D, Honore C, et al. Value of preoperative staging in patients with peritoneal carcinomatosis. J Surg Oncol. 2005;91(4):213-219.
[12] Sugarbaker PH. Cytoreductive surgery and perioperative chemotherapy for peritoneal surface malignancy: rationale and results. Eur J Surg Oncol. 2001;27(6):593-599.
[13] Laghi A, Catalano C, Iannicelli E, et al. Peritoneal carcinomatosis: imaging diagnosis. Abdom Imaging. 2005;30(2):145-157.
[14] Forstner R, Hricak H, Thomassin-Naggara I. Imaging of peritoneal carcinomatosis: current status and future directions. Radiology. 2011;260(2):347-359.
[15] Yang HK, Kim JW, Kim MS, et al. Preoperative staging of gastric cancer with peritoneal carcinomatosis using contrast-enhanced ultrasound. World J Gastroenterol. 2015;21(35):10135-10144.
[16] Yu J, Turner MA, Fulcher AS, et al. Peritoneal metastases: spectrum of appearances and pitfalls in diagnosis at US. Radiographics. 2006;26(5):1651-1672.
[17] Cibula D, Vlasák P, Fischerová D, et al. Accuracy of preoperative transvaginal and transabdominal ultrasound in predicting rectosigmoid infiltration in patients with advanced ovarian cancer. Ultrasound Obstet Gynecol. 2002;20(2):173-178.
[18] Jager GJ, de Wilt JH, Hermans J, et al. Detection of peritoneal carcinomatosis with spiral computed tomography and magnetic resonance imaging: a prospective study. Eur J Radiol. 2000;34(3):197-207.
[19] Guinet C, Ghouti L, Koh DM, et al. Peritoneal carcinomatosis: evaluation with diffusion-weighted MR imaging. Radiology. 2011;261(1):143-150.
[20] Even-Sapir E, Lerman H, Gutman M, et al. 18F-FDG PET/CT in evaluation of suspected recurrent ovarian cancer: comparison with conventional workup. Radiology. 2006;238(3):933-945.
[21] Hricak H, Vargas HA, Chung S, et al. Combined PET/MR imaging in oncology: clinical applications and challenges. Radiology. 2011;258(3):661-680.
[22] Park SJ, Kim SH, Kim DY, et al. Comparison of CT, MRI, and PET/CT for detecting peritoneal carcinomatosis. Abdom Imaging. 2013;38(5):984-991.
[23] Low RN, Semelka RC, Hatabu H, et al. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) in patients with peritoneal carcinomatosis from ovarian cancer. J Magn Reson Imaging. 2008;27(5):1053-1062.
[24] Van Ramshorst B, Brandwijk M, de Wilt JH, et al. Value of computed tomography and magnetic resonance imaging in selection of patients with colorectal peritoneal carcinomatosis for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol. 2008;15(11):3064-3071.
[25] Van Der Speeten K, Stuart-Smith C, Tummers P, et al. The accuracy of computed tomography scan and laparoscopy in predicting complete cytoreduction in patients with colorectal peritoneal carcinomatosis. J Surg Oncol. 2011;103(7):637-641.
[26] Forstner R, Sala E, Thoeny HC. ESUR guidelines: ovarian cancer staging and follow-up. Eur Radiol. 2010;20(12):2773-2780.
[27] Elias D, Goéré D, Blot F, et al. Peritoneal carcinomatosis from colorectal cancer: evolution in survival after complete cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 2003;238(2):265-269.
[28] Sugarbaker PH. Peritoneal surface oncology: history, controversies, and the future. Ann Surg Oncol. 2005;12(10):811-822.
[29] Ceelen WP, Bracke ME, Rillaerts E, et al. Intraperitoneal chemotherapy for colorectal peritoneal carcinomatosis: a systematic review. Ann Surg Oncol. 2008;15(5):1257-1269.
[30] Yonemura Y, Elnemr A, Endou Y, et al. Bidirectional chemotherapy combined with cytoreductive surgery for peritoneal carcinomatosis from gastric cancer: a phase III randomized controlled study. Ann Surg Oncol. 2008;15(11):3047-3055.
[31] Glehen O, Gilly FN, Arvieux C, et al. Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol. 2010;17(9):2370-2377.
[32] Sugarbaker PH. Peritonectomy procedures. In: Sugarbaker PH, editor. Surgical Treatment of Peritoneal Surface Malignancy for the Clinician. Richmond, BC: CoGraphics; 2007. pp. 121–144.
