Introduction
Soil-transmitted helminths, including Ascaris lumbricoides, Trichuris trichiura, and hookworm, pose a significant global health challenge, affecting approximately 2 billion people worldwide. These parasitic infections are particularly prevalent in developing countries, especially in rural areas lacking adequate sanitation infrastructure.1 Children are disproportionately vulnerable, with chronic infections leading to anemia, malnutrition, and impaired physical and cognitive development.2
Traditional diagnosis of soil-transmitted helminths relies on microscopic examination of stool samples.3 The Kato–Katz method is a commonly employed technique,4 and its sensitivity can be enhanced through repeated stool examinations.5 However, the need for novel diagnostic approaches is critical, as many infected individuals reside in underserved rural communities with limited access to diagnostic facilities and healthcare.2 This article explores the innovative concept of transforming a mobile phone into a microscope for point-of-care diagnosis of soil-transmitted helminths. We present a proof-of-concept study comparing the diagnostic accuracy of our mobile phone microscope with conventional light microscopy, highlighting the potential of Phone Diagnosis in global health.
Methods
Ethics Statement
This proof-of-concept study was embedded within a larger clinical trial evaluating the safety and efficacy of anthelmintic drugs against soil-transmitted helminth infections in school children on Pemba Island, Tanzania. Ethical approvals were obtained from the ethics committee of Basel (EKBB; reference no. 390/11) and the Ministry of Health and Social Welfare of Zanzibar (ZAMREC; reference no. 0001/Jan/11). The clinical trial was registered with ClinicalTrials.gov (identifier ISRCTN54577342). Written informed consent was obtained from parents or legal guardians, and oral assent was obtained from the children. All participants received albendazole (400 mg) treatment at the study’s conclusion.
Procedures
The clinical trial took place during September and October 2012. For this specific study, stool samples were collected from children each morning over five consecutive days. Samples were processed on the same day using the Kato–Katz technique.4 Conventional light microscopy was performed by trained laboratory technicians to analyze stool samples, and eggs of A. lumbricoides, T. trichiura, and hookworm were counted and recorded separately. Data were double-entered into a database to minimize errors. Quality control was maintained by re-examining a random 10% of slides by a senior microscopist to ensure internal validity and accuracy of the initial diagnoses.
Mobile Phone Microscope: A Tool for Phone Diagnosis
We developed a mobile phone microscope by attaching a 3-mm ball lens (Edmund Optics, Barrington, NJ) to the camera of an iPhone 4S (Apple, Cupertino, CA) using double-sided tape (3M, St. Paul, MN). This method was inspired by the work of Smith and colleagues.6 A small aperture was created in the double-sided tape, and the ball lens was positioned within this aperture. The ball lens was then carefully centered over the iPhone camera lens and secured with the tape to ensure stability. Kato–Katz thick smear slides were placed directly against the double-sided tape, maintaining a small gap of less than 1 mm between the lens and the slide (Figure 1). Illumination was provided from below the slide using a generic, small, hand-held incandescent flashlight powered by a single AA battery. Images were viewed on the iPhone screen, and digital zoom was used to increase magnification, achieving an estimated magnification of 50–60×. The microscopist manually moved the slide beneath the mobile phone microscope to examine the entire stool area on the slide, facilitating comprehensive phone diagnosis. The thick double-sided tape (3M) acted as a 1-mm buffer, preventing direct contact between the slide and ball lens. Furthermore, the cellophane strip covering the stool on the slide prevented stool contamination of the ball lens, ensuring consistent image quality for phone diagnosis.
Figure 1.
Samples for Mobile Phone Diagnosis
A total of 199 Kato–Katz thick smear slides were randomly selected and analyzed using mobile phone microscopy by a senior microscopist. This analysis was performed within 2 hours of the conventional light microscopy evaluation. The microscopist using the mobile phone microscope was blinded to the results obtained from conventional light microscopy to ensure unbiased assessment of phone diagnosis accuracy.
Statistical Analysis
Data were analyzed using Stata version 10.1 (StataCorp., College Station, TX). We calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of mobile phone microscopy in diagnosing soil-transmitted helminths, comparing these metrics to results from conventional microscopy. While a definitive ‘gold standard’ for diagnosing soil-transmitted helminths is lacking, microscopy on multiple samples is generally considered a robust diagnostic standard. Comparisons were made between the detection of any helminth egg by mobile phone microscopy and conventional microscopy, irrespective of species identification accuracy. We also evaluated the diagnostic accuracy of phone diagnosis for each soil-transmitted helminth species individually. Additionally, diagnostic accuracy was assessed for low, moderate, and heavy infection intensities, using World Health Organization (WHO) cutoffs.7 Moderate and heavy infection intensities were combined into a single category for analysis. Due to the selection of positive slides for evaluating low and moderate/heavy infection intensities, specificity, PPV, and NPV could not be assessed for these categories, as there were no false-positive slides from conventional microscopy in these selected samples.
Results
A total of 199 Kato–Katz thick smears were analyzed by both mobile phone microscopy and conventional microscopy. Table 1 summarizes the sensitivity, specificity, PPV, and NPV for soil-transmitted helminth diagnosis using phone diagnosis. Notably, the mobile phone microscope demonstrated a sensitivity of 69.4% for detecting any soil-transmitted helminth infection. The highest sensitivity was observed for A. lumbricoides (81.0%), followed by T. trichiura (54.4%). The sensitivity for hookworm detection was considerably lower (14.3%). The mobile phone microscope showed improved sensitivity for heavy infection intensities compared to lower fecal egg counts. In this study, all hookworm infections were of low intensity (Figure 2).
Table 1.
Operating characteristics of the mobile phone microscope compared with conventional microscopy for the diagnosis of soil-transmitted helminth infection (n = 199 Kato-Katz thick smears)
| Organism | Conventional microscope n (%) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Any soil-transmitted helminth egg visualized | 173 (86.9) | 69.4 (61.8–76.0) | 61.5 (40.7–79.1) | 92.3 (85.9–96.0) | 23.2 (14.2–35.2) |
| All A. lumbricoides | 41 (20.6) | 81.0 (65.4–90.9) | 87.3 (80.7–91.9) | 63.0 (48.7–75.4) | 94.5 (89.1–97.4) |
| A. lumbricoides (light infection)* | 27 (13.6) | 74.5 (53.4–88.1) | – | – | – |
| A. lumbricoides (moderate/heavy infection)† | 15 (7.5) | 93.3 (66.0–99.7) | – | – | – |
| All T. trichiura | 158 (79.4) | 54.4 (46.3–62.3) | 63.4 (46.9–77.4) | 85.1 (76.4–91.2) | 26.5 (18.4–36.6) |
| T. trichiura (light infection)‡ | 107 (53.8) | 43.9 (34.5–53.8) | – | – | – |
| T. trichiura (moderate/heavy infection)§ | 51 (25.6) | 76.5 (62.2–86.7) | – | – | – |
| All hookworm¶ | 98 (49.2) | 14.3 (8.3–23.1) | 89.1 (81.0–94.2) | 56.0 (35.3–75.0) | 51.7 (44.1–59.3) |

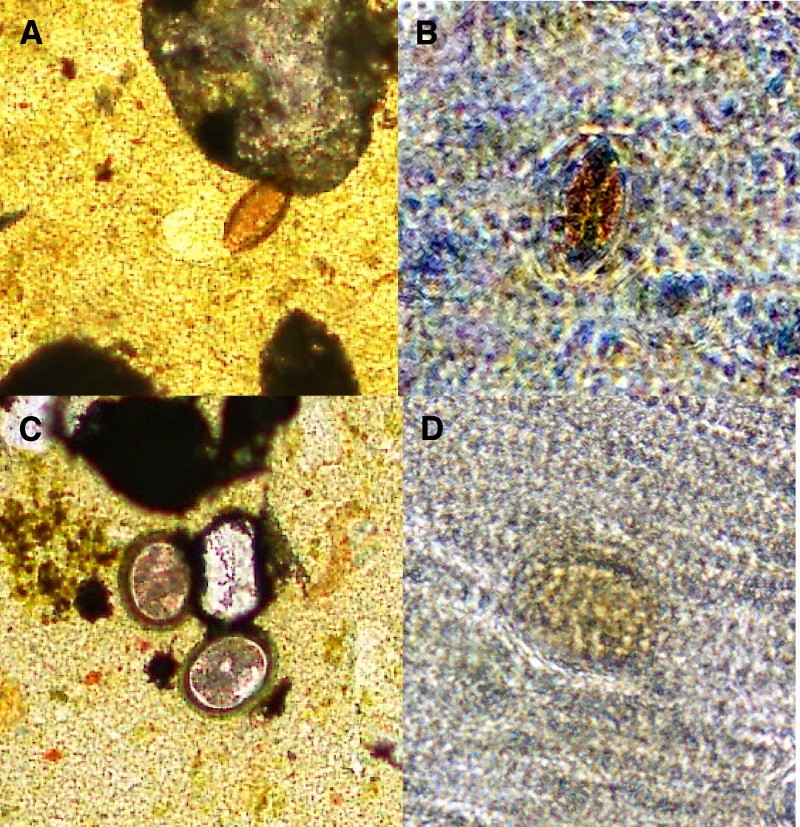
Figure 2.
Soil-transmitted helminth eggs visualized by conventional and mobile phone microscopy. T. trichiura is shown at 40× magnification by conventional microscopy in A and mobile phone microscopy in B. A. lumbricoides is shown at 40× magnification by conventional microscopy in C and mobile phone microscopy in D.
Discussion
This study provides a proof-of-concept for a first-generation mobile phone light microscope as a tool for point-of-care diagnosis of soil-transmitted helminth infections in school-aged children on Pemba Island, a region known for high prevalence of multiple helminth species.8 While our simple mobile phone microscope achieved only moderate diagnostic accuracy compared to conventional light microscopy, particularly in overall sensitivity, it showed promising results for moderate to heavy A. lumbricoides infections. We believe mobile phone microscopy represents a promising innovation for point-of-care intestinal helminth diagnosis due to its portability, ease of assembly and use, and low cost. Crucially, mobile phones are now globally ubiquitous.9 These affordable devices are robust in field conditions, and their prices continue to decrease, making phone diagnosis increasingly accessible. At the time of this publication, a used iPhone 4S was priced at around US$250, with prices expected to decline further as newer models become available, enhancing the feasibility of widespread adoption of phone diagnosis tools.
The modest diagnostic performance of this first-generation mobile phone microscope for soil-transmitted helminth diagnosis can be attributed to several factors. Firstly, the image quality produced by our 3-mm ball lens microscope is not as sharp as that of conventional microscopy. While smaller lenses offer higher resolution, they also have a narrower field of view, as observed with 1-mm ball lenses used in laboratory mobile phone microscopes.6 We opted for a 3-mm ball lens to prioritize a larger field of view over higher resolution, aiming to scan the entire Kato–Katz thick smear for helminth eggs in field settings, where clinicians need to quickly determine egg presence anywhere on the slide for effective phone diagnosis. Improved image quality has been reported using devices that mount mobile phones onto conventional light microscopes; however, such setups are not practical for portable point-of-care testing.10 Although helminth eggs can be reliably visualized with our mobile phone microscope, accurate species identification remains challenging. Additionally, the mobile phone microscope occasionally detected false-positive eggs, likely due to stool artifacts caused by the relatively lower image quality inherent in early phone diagnosis technology. T. trichiura eggs, being smaller than A. lumbricoides eggs, were more challenging to detect with mobile phone microscopy, contributing to the lower sensitivity observed for this helminth species in phone diagnosis scenarios. Hookworm diagnosis was particularly poor; however, it is important to note that hookworm eggs are known to clear rapidly using the Kato–Katz method,11 and despite our efforts for rapid analysis, this rapid clearing combined with lower image quality made hookworm detection difficult with phone diagnosis in this setup.
From a practical perspective, the sensitivity of approximately 70% for detecting any soil-transmitted helminth egg using mobile phone microscopy is promising, although not yet sufficient for immediate widespread clinical application. However, it approaches clinically relevant diagnostic characteristics. For instance, if a clinical decision hinges on determining whether a patient has any soil-transmitted helminth infection, the detection of any egg, regardless of species, should prompt treatment with a broad-spectrum anthelmintic drug. Furthermore, appropriate laboratory confirmation should be sought to ensure the selection of the most effective anthelmintic drug and to guide subsequent follow-up care, particularly when assessing treatment response. It is important to note that albendazole is ineffective against Schistosoma mansoni and exhibits only moderate efficacy against T. trichiura when administered as a single dose.12 Significantly, co-infection with multiple helminth species is common in much of sub-Saharan Africa.2,13,14 S. mansoni eggs are considerably larger than soil-transmitted helminth eggs and possess distinct morphological features (e.g., a lateral spine), making them readily distinguishable from soil-transmitted helminth eggs even with mobile phone diagnosis, based on our experience. Mobile phone microscopy is likely to become clinically highly valuable when sensitivities reach or exceed 80%, enhancing its utility in point-of-care settings.
Our choice of a simple, first-generation ball-lens mobile phone microscope was driven by its ease of construction and usability. This device can be assembled in under 5 minutes at an approximate cost of US$15, making it highly attractive for resource-constrained environments where affordable diagnostic tools are urgently needed for effective phone diagnosis strategies. However, various other cell phone microscopy methods are under development that promise improved resolution. Next-generation technologies, such as lens-free devices, may offer exceptional diagnostic capabilities15–17; however, these methods require further field testing. Other light-microscope attachments for mobile phones can also generate superior image quality, likely enhancing diagnostic accuracy for phone diagnosis applications.18 These advanced technologies hold significant potential to serve as effective point-of-care diagnostic tools in resource-limited settings,9 but rigorous field testing outside controlled laboratory environments is essential to validate their diagnostic utility and practicality for widespread implementation of phone diagnosis. Additionally, the digitization of images facilitated by mobile phone diagnosis can aid in diagnoses by enabling clinicians to transmit images to centralized locations for expert diagnostic support and remote consultations, enhancing healthcare accessibility in remote regions.10,19
Mobile phone diagnostic technology is poised to significantly contribute to the diagnosis of both infectious and non-infectious diseases in resource-limited settings. While a first-generation ball-lens mobile phone microscope demonstrated modest diagnostic yield for soil-transmitted helminth infections, newer technologies are continually improving mobile phone diagnosis capabilities. Further field testing in diverse epidemiological settings is crucial to fully realize the potential of mobile phone diagnosis in global health.
Disclaimer: The funders (Medicor Foundation and SNSF) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by The Medicor Foundation. J.K. is financially supported by the Swiss National Science Foundation (SNSF; projects PPOOA-114941 and PPOOP3_135170).
Authors’ addresses: Isaac I. Bogoch, Divisions of Internal Medicine and Infectious Diseases, Toronto General Hospital, Toronto, Ontario, Canada, E-mail: [email protected]. Jason R. Andrews, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, E-mail: [email protected]. Benjamin Speich, Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: [email protected]. Jürg Utzinger, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: [email protected]. Shaali M. Ame and Said M. Ali, Public Health Laboratory, Ivo de Carneri, Pemba Island, Zanzibar, United Republic of Tanzania, E-mails: [email protected] and [email protected]. Jennifer Keiser, Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: [email protected].
