Introduction to Pleural Effusion and its Significance
Pleural effusion, defined as the abnormal accumulation of fluid within the pleural space, is a frequently encountered clinical entity with a broad spectrum of underlying etiologies. In routine medical practice, the precise identification of the cause of a pleural effusion is paramount. This is because the etiology directly dictates the subsequent treatment strategies and prognostic implications for the patient. From benign conditions to life-threatening malignancies, pleural effusions represent a diagnostic challenge that necessitates a systematic and thorough approach.
It is estimated that hundreds of thousands of individuals are affected by pleural effusion annually. The causes are diverse, ranging from relatively benign effusions associated with viral pleuritis to serious conditions like congestive heart failure or malignancy. The clinical significance is underscored by the substantial mortality associated with non-malignant pleural effusions, with reported one-year mortality rates ranging from 25% to 57%. Consequently, determining the underlying cause of a pleural effusion is not merely an academic exercise but a critical step in guiding appropriate therapy and improving patient outcomes.
The diagnostic journey for pleural effusion often begins with clinical assessment and imaging, but definitive diagnosis frequently hinges on the analysis of pleural fluid obtained via thoracentesis. This review aims to provide a comprehensive overview of Pleural Fluid Diagnosis, emphasizing the crucial role of pleural fluid analysis in differentiating various etiologies and guiding clinical decision-making. We will explore the principles of pleural fluid physiology, the clinical presentation of pleural effusions, the step-by-step approach to pleural fluid analysis, and the interpretation of diagnostic findings.
Understanding Pleural Fluid: Physiology and Pathophysiology
A fundamental understanding of pleural fluid physiology is essential for interpreting diagnostic findings and comprehending the mechanisms underlying pleural effusion formation. The pleural space, situated between the visceral and parietal pleura, normally contains a small volume of fluid that acts as a lubricant, facilitating frictionless lung movement during respiration. This fluid is in a dynamic state of equilibrium, with continuous production and absorption maintaining a delicate balance.
Both the visceral and parietal pleura contribute to pleural fluid homeostasis. Under normal physiological conditions, the production and absorption rates are approximately 0.2 mL/kg/hr, resulting in a complete turnover of pleural fluid volume within an hour. The parietal pleura is primarily responsible for both fluid production and resorption. An exception to this general rule is pleural effusion secondary to left-heart failure, where the fluid originates predominantly from the visceral pleura.
The volume of pleural fluid is determined by the intricate interplay of hydrostatic and oncotic pressure gradients between the systemic and pulmonary circulation and the pleural space. Fluid resorption occurs primarily through lymphatic vessels located in the parietal pleura. Remarkably, the lymphatic system possesses a significant reserve capacity, capable of increasing its flow rate by up to 20-fold in response to increased pleural fluid production.
Pleural effusion arises when this equilibrium is disrupted, typically due to either increased fluid production, decreased fluid resorption, or a combination of both. Several pathophysiological mechanisms can contribute to this imbalance, including:
- Reduced oncotic pressure: Hypoalbuminemia, for instance, decreases the oncotic pressure within capillaries, favoring fluid transudation into the pleural space.
- Elevated pulmonary capillary pressure: Congestive heart failure increases pulmonary capillary hydrostatic pressure, driving fluid out of capillaries and into the pleural space.
- Increased pleural membrane permeability: Inflammation or injury to the pleura can increase its permeability, allowing excessive fluid and protein to leak into the pleural space.
- Lymphatic obstruction: Impaired lymphatic drainage, due to malignancy or other causes, can hinder fluid resorption from the pleural space.
- Diminished negative intrapleural pressure: Conditions that reduce the negative pressure within the pleural space can also contribute to fluid accumulation.
These pathophysiological derangements ultimately lead to the clinical manifestation of pleural effusion, which can be broadly categorized as transudates or exudates based on pleural fluid analysis, a distinction that is crucial for differential diagnosis.
Clinical Presentation of Pleural Effusion
The clinical manifestations of pleural effusion are highly variable and often depend on the underlying etiology and the size of the effusion. Many patients, particularly those with small effusions, may be asymptomatic, and the effusion may be detected incidentally on chest imaging performed for other reasons. When symptoms do occur, they can be attributed to pleural inflammation, impaired pulmonary mechanics, or disturbances in gas exchange.
Dyspnea, or shortness of breath, is the most frequently reported symptom associated with pleural effusion. However, the severity of dyspnea does not always correlate directly with the size of the effusion. Large effusions can compress lung tissue, reducing lung volumes and contributing to dyspnea. Interestingly, even after draining a large effusion, lung volumes may not immediately improve. The relief of dyspnea following drainage is likely related to improved respiratory muscle mechanics, particularly optimizing the length-tension relationship of the diaphragm.
Pleuritic chest pain, a sharp, localized pain exacerbated by breathing or coughing, is another common symptom, especially in inflammatory pleural effusions. This pain arises from irritation of the parietal pleura, which is rich in nociceptors, unlike the visceral pleura. Pleuritic pain often diminishes or resolves once a pleural effusion develops, potentially due to the separation of the inflamed pleural surfaces by the accumulating fluid.
Some patients may experience a dull, oppressive chest discomfort, especially when the parietal pleura is directly involved in the pathological process, such as in empyema, primary pleural malignancies, or pleural carcinomatosis. Effusions in these scenarios are typically exudative in nature.
Other less common symptoms include a dry cough, which may be triggered by pleural inflammation or lung compression, and sleep disturbances, potentially due to nocturnal dyspnea or discomfort.
A comprehensive clinical history is crucial in evaluating pleural effusion. Key aspects of the history include:
- Recent respiratory infections: To assess for parapneumonic effusions.
- Fever, weight loss, malaise: Suggestive of infectious or malignant etiologies.
- Temporal course of symptoms: Rapid onset may indicate acute conditions like pulmonary embolism, while gradual onset may suggest chronic processes.
- Pre-existing chronic illnesses: Particularly heart disease, liver disease, or kidney disease, which are common causes of transudative effusions.
- History of malignancy: To evaluate for malignant pleural effusion.
- Pleuritic chest pain: Suggestive of pulmonary embolism or inflammatory conditions.
- Medication history: Certain drugs can induce pleural effusions.
- Asbestos exposure: A risk factor for mesothelioma.
Physical examination findings may include diminished or absent breath sounds and dullness to percussion over the affected hemithorax, particularly at the lung bases. Tachypnea may be present with large effusions. A pleural friction rub, a coarse scratching sound, may be auscultated in the early stages of parapneumonic effusions. The presence of peripheral edema, jugular venous distention, and a third heart sound may suggest congestive heart failure as the underlying cause, particularly in bilateral effusions. Ascites in a patient with cirrhosis raises suspicion for hepatic hydrothorax.
While clinical history and physical examination provide valuable clues, imaging studies are essential for confirming the presence of pleural effusion and guiding further diagnostic and therapeutic interventions.
Diagnostic Evaluation: Imaging and Clinical Assessment
When pleural effusion is suspected based on clinical presentation, imaging studies play a crucial role in confirming the diagnosis, estimating the size and location of the effusion, and guiding further diagnostic procedures. Chest radiography is typically the initial imaging modality.
Posteroanterior (PA) chest radiographs can detect effusions of 200 mL or greater, while lateral views can identify smaller effusions, as small as 50 mL. Lateral decubitus views, obtained with the patient lying on their side, can confirm the free-flowing nature of the effusion and differentiate it from loculated effusions or pleural thickening.
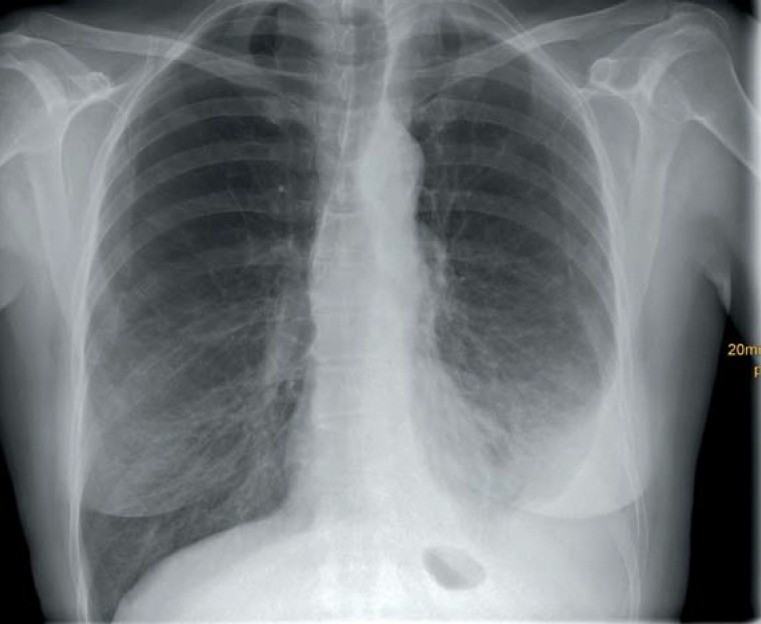 Chest x-ray of a 59-year-old woman with a left-sided pleural effusion. Further work-up revealed a pleural mesothelioma as the cause.
Chest x-ray of a 59-year-old woman with a left-sided pleural effusion. Further work-up revealed a pleural mesothelioma as the cause.
Figure 1: Chest radiograph demonstrating a left pleural effusion in a 59-year-old female patient, later diagnosed with pleural mesothelioma.
Chest ultrasound is a highly valuable tool in pleural effusion evaluation. It is more sensitive than chest CT in detecting pleural septations, which is particularly important when multiple thoracenteses may be required. Ultrasound guidance during thoracentesis significantly reduces the risk of iatrogenic pneumothorax. Ultrasound is especially useful in critically ill or ventilated patients in the supine position, where chest radiographs are less sensitive.
Chest computed tomography (CT) is more sensitive than plain radiography in detecting small pleural effusions and can differentiate pleural fluid from pleural tissue thickening. CT scans can also provide clues to the underlying etiology, such as pneumonia, malignancy, or pulmonary embolism. Ideally, chest CT should be performed after initial thoracentesis and drainage, as the effusion itself can obscure underlying pleural and pulmonary pathology. Contrast-enhanced CT is particularly helpful in diagnosing pleural empyema and delineating lung abscesses. While CT can suggest malignancy, it cannot reliably differentiate pleural carcinomatosis from mesothelioma.
Clinical assessment, combined with imaging findings, can sometimes strongly suggest the nature of the pleural effusion. For instance, a patient with clinical signs of congestive heart failure, such as peripheral edema, tachycardia, and bilateral basilar dullness to percussion, is highly likely to have a transudative pleural effusion of cardiac origin. In such cases, diagnostic thoracentesis may be deferred, and treatment directed at the underlying heart failure. Similarly, in a patient with known hepatic cirrhosis and ascites presenting with bilateral pleural effusions, hepatic hydrothorax is the likely diagnosis.
However, in many cases, particularly with unilateral pleural effusions or when the etiology is uncertain, further diagnostic evaluation, including pleural fluid analysis, is necessary.
Thoracentesis: The Cornerstone of Pleural Fluid Diagnosis
Thoracentesis, the procedure of aspirating pleural fluid from the pleural space, is a central component of pleural fluid diagnosis. Pleural fluid analysis is essential for differentiating transudates from exudates and for identifying specific etiologies of pleural effusion.
Indications for Thoracentesis
Diagnostic thoracentesis is indicated in nearly all patients with pleural effusion of unclear etiology. Specifically, thoracentesis should be performed when:
- The cause of pleural effusion is not readily apparent from clinical history and non-invasive investigations.
- Unilateral pleural effusion is present.
- Atypical features are present in bilateral effusions, such as pleuritic chest pain or disproportionate symptoms.
- There is a lack of expected response to treatment for a presumed underlying condition.
- Empyema is suspected in the context of pneumonia.
Therapeutic thoracentesis, involving the removal of larger volumes of pleural fluid, is indicated to alleviate symptoms, particularly dyspnea, caused by large pleural effusions. It may also be necessary in cases of large effusions causing respiratory or cardiac compromise.
In patients with bilateral pleural effusions and a clear underlying diagnosis such as congestive heart failure, diagnostic thoracentesis may not be routinely necessary, provided the clinical presentation is typical and there is a response to treatment of the underlying condition. However, if atypical features are present or the response to treatment is suboptimal, diagnostic thoracentesis should be considered.
Performing Thoracentesis: Procedure and Best Practices
Thoracentesis should ideally be performed under ultrasound guidance, which improves safety and success rates, especially for smaller effusions or loculated fluid. Ultrasound guidance minimizes the risk of complications, particularly pneumothorax.
Thoracentesis is typically performed with the patient sitting upright, leaning forward to maximize the intercostal space. After identifying the optimal puncture site, usually in the mid-axillary or posterior axillary line, the skin is prepped and draped in a sterile fashion. Local anesthesia is administered. A small-gauge needle (e.g., 21-gauge) attached to a syringe, often with a three-way stopcock, is inserted into the pleural space. Commercially available thoracentesis kits can simplify the procedure.
For diagnostic purposes, approximately 50 mL of pleural fluid is usually sufficient. For therapeutic thoracentesis, larger volumes may be removed, but it is generally recommended to limit aspiration to 1-1.5 liters at a time to minimize the risk of re-expansion pulmonary edema.
If pleural fluid pH measurement is required, a heparinized blood gas syringe should be used, and the sample should be analyzed promptly. Collected pleural fluid should be divided into aliquots for various analyses, including:
- Microbiology: 5 mL for Gram stain, bacterial culture (aerobic and anaerobic), and fungal or mycobacterial cultures if indicated. Blood culture bottles can enhance bacterial pathogen detection, particularly for anaerobes, but are not recommended for Mycobacterium tuberculosis.
- Biochemistry: 2-5 mL for protein, lactate dehydrogenase (LDH), glucose, amylase, cholesterol, and other tests as clinically indicated.
- Cytology: 20-40 mL for cell count and differential, and cytopathological examination to detect malignant cells.
Thoracentesis, while generally safe, carries a risk of complications, the most common being pneumothorax. The risk of pneumothorax ranges from 0.6% to 6%. Post-procedure monitoring for 1-4 hours is recommended, as pneumothorax typically becomes clinically apparent within this timeframe. Routine post-thoracentesis chest radiography is generally not necessary in asymptomatic patients without new respiratory symptoms.
Thoracentesis under ultrasound guidance is particularly valuable in intensive care settings, especially for ventilated patients and for evaluating small effusions of unknown origin. Elective thoracentesis should be performed during regular working hours to minimize procedure-related risks. Coagulation parameters should be assessed, and ideally, the INR should be less than 1.5 before the procedure.
Pleural Fluid Analysis: Unlocking Diagnostic Insights
Analysis of pleural fluid is the cornerstone of diagnosing the etiology of pleural effusion. Pleural fluid analysis encompasses macroscopic examination, biochemical tests, cytological evaluation, and microbiological studies.
Macroscopic Examination of Pleural Fluid
The gross appearance of pleural fluid can provide immediate clues to the diagnosis:
- Milky fluid: Suggestive of chylothorax, indicating lymphatic leakage.
- Purulent fluid (pus): Diagnostic of empyema, indicating infection within the pleural space.
- Bloody fluid: May indicate malignancy, trauma, pulmonary embolism, or iatrogenic puncture. However, a bloody tap can also be due to traumatic needle insertion.
Centrifugation can help differentiate chylothorax from empyema. Chylous fluid remains milky after centrifugation, while empyema fluid typically shows a clear supernatant.
Differentiating Transudates from Exudates: Light’s Criteria and Beyond
The initial step in pleural fluid analysis is to classify the effusion as either a transudate or an exudate. This distinction is fundamental as it significantly narrows the differential diagnosis and guides further investigations. Light’s criteria are the most widely used and validated criteria for this differentiation.
Light’s Criteria for Exudative Pleural Effusion:
Pleural effusion is classified as exudative if at least one of the following criteria is met:
- Pleural fluid protein to serum protein ratio > 0.5
- Pleural fluid LDH to serum LDH ratio > 0.6
- Pleural fluid LDH > two-thirds of the upper limit of normal for serum LDH (typically > 200 IU/L)
If none of these criteria are met, the effusion is classified as a transudate. Light’s criteria have a high sensitivity (99.5%) and specificity (93-96%) for identifying exudates.
Transudates typically result from systemic factors that alter hydrostatic or oncotic pressures, such as:
- Congestive heart failure (most common cause of transudative effusion)
- Hepatic cirrhosis with hepatic hydrothorax
- Nephrotic syndrome
- Pulmonary embolism (can be transudate or exudate)
- Hypoalbuminemia
Exudates, on the other hand, are usually caused by local factors affecting the pleura or lung, such as:
- Infections (pneumonia, empyema, tuberculosis)
- Malignancy (lung cancer, breast cancer, mesothelioma, lymphoma)
- Pulmonary embolism (can be transudate or exudate)
- Inflammatory conditions (rheumatoid arthritis, systemic lupus erythematosus)
- Gastrointestinal diseases (pancreatitis, esophageal perforation)
It is important to note that diuretics administered for congestive heart failure can alter pleural fluid composition, potentially leading to misclassification of a transudate as an exudate (“pseudoexudate”). Pleural fluid analysis should ideally be performed before diuretic therapy is initiated or interpreted cautiously in patients already on diuretics. In cases of suspected pseudoexudate, measuring the serum-pleural fluid protein gradient can be helpful. A gradient greater than 3.1 g/dL suggests a transudate despite meeting exudative criteria by Light’s criteria.
Advanced Pleural Fluid Tests: pH, Glucose, Amylase, and Biomarkers
In addition to protein and LDH, other biochemical tests on pleural fluid can provide valuable diagnostic information:
- pH: Pleural fluid pH is crucial in parapneumonic effusions and empyema. A pH < 7.2 in a parapneumonic effusion indicates a complicated effusion and often necessitates chest tube drainage. Pleural fluid acidosis (low pH) can also be seen in tuberculous pleuritis, rheumatoid pleuritis, and malignant pleural effusions. In malignant effusions, a lower pH is associated with poorer prognosis and lower pleurodesis success rates.
- Glucose: Pleural fluid glucose concentration is normally similar to serum glucose. Low pleural fluid glucose (< 60 mg/dL) is seen in empyema, tuberculous pleuritis, rheumatoid pleuritis, and malignant pleural effusions. In empyema and rheumatoid effusions, it can be very low (< 30 mg/dL).
- Amylase: Elevated pleural fluid amylase is suggestive of pancreatitis or esophageal rupture. In pancreatitis-associated effusions, amylase levels are typically several times higher than serum amylase levels.
- NT-proBNP: N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a biomarker for heart failure. Elevated pleural fluid NT-proBNP levels strongly suggest congestive heart failure as the cause of pleural effusion, even if the effusion appears exudative by Light’s criteria. Measuring serum NT-proBNP is often sufficient, and a negative serum NT-proBNP effectively rules out heart failure as the cause.
- Cholesterol and Triglycerides: Measurement of cholesterol and triglycerides is helpful in suspected chylothorax or pseudo-chylothorax (cholesterol effusion). A pleural fluid cholesterol level > 55 mg/dL combined with LDH > 200 U/L increases the specificity for exudate. Pleural fluid triglyceride levels > 110 mg/dL are highly suggestive of chylothorax.
Cytological and Microbiological Examination of Pleural Fluid
Cytological and microbiological examinations of pleural fluid are essential for identifying malignant and infectious etiologies.
- Cell count and differential: Pleural fluid cell counts and differential can provide clues to the etiology. Neutrophil-predominant effusions are often seen in acute processes like parapneumonic effusions, empyema, and pulmonary embolism. Lymphocyte-predominant effusions are more common in tuberculous pleuritis, malignant effusions, and chronic effusions. However, cell counts alone are not definitive for diagnosis.
- Cytology: Cytological examination of pleural fluid is crucial for diagnosing malignant pleural effusion. Cytology is positive in approximately 50% of lung cancer-related malignant effusions and in about 60% of all malignant effusions. Adenocarcinoma has the highest yield, while mesothelioma, squamous cell carcinoma, lymphoma, and sarcoma have lower yields. A large volume (20-60 mL) of fluid should be submitted for cytology. Histological examination is often necessary for mesothelioma diagnosis.
- Gram stain and culture: Gram stain and bacterial culture are essential in suspected parapneumonic effusions and empyema. Gram stain can provide rapid preliminary identification of bacteria. Blood culture bottles enhance bacterial detection, especially for anaerobes. Microbiological yield in non-purulent parapneumonic effusions is only about 25%, and false negatives are common. PCR-based techniques targeting the 16S rRNA gene can improve sensitivity.
- Mycobacterial studies: In suspected tuberculous pleuritis, pleural fluid should be sent for acid-fast bacilli (AFB) stain and mycobacterial culture. Large volumes (30-50 mL) of fresh, untreated fluid should be submitted. AFB stain has low sensitivity (< 5%), and culture sensitivity is also limited (10-20%). PCR for Mycobacterium tuberculosis DNA may be helpful but can be affected by inhibitors in pleural fluid. Pleural biopsy is often necessary for diagnosing tuberculous pleuritis.
Diagnostic Algorithm for Pleural Effusion
The diagnostic approach to pleural effusion should be systematic and stepwise, integrating clinical assessment, imaging, and pleural fluid analysis. A practical diagnostic algorithm can be summarized as follows (refer to Figure 2 in the original article for a visual representation):
- Clinical Assessment and Chest Radiography: Evaluate clinical history, physical examination, and chest radiograph to confirm pleural effusion and assess for potential underlying causes (e.g., heart failure, pneumonia, malignancy).
- Thoracentesis and Pleural Fluid Analysis: If the etiology is unclear or exudative effusion is suspected, perform diagnostic thoracentesis. Analyze pleural fluid for:
- Macroscopic appearance
- Protein and LDH (Light’s criteria to differentiate transudate vs. exudate)
- Cell count and differential
- Gram stain and bacterial culture (if infection suspected)
- Cytology (to evaluate for malignancy)
- Further Pleural Fluid Tests (as indicated): Based on initial pleural fluid analysis and clinical suspicion, consider additional tests:
- pH and glucose (if parapneumonic effusion or empyema suspected)
- Amylase (if pancreatitis or esophageal rupture suspected)
- NT-proBNP (if heart failure suspected, even with exudative effusion)
- Mycobacterial studies (AFB stain, culture, PCR) if tuberculous pleuritis suspected
- Cholesterol and triglycerides (if chylothorax or pseudo-chylothorax suspected)
- Imaging and Invasive Procedures (if needed): If pleural fluid analysis is non-diagnostic or malignancy is highly suspected:
- Chest CT (if not already performed) to further evaluate lung parenchyma, pleura, and mediastinum.
- Pleural biopsy (image-guided needle biopsy or thoracoscopic biopsy) if malignancy or tuberculous pleuritis is suspected and cytology/mycobacterial studies are negative.
- Thoracoscopy (VATS) for direct pleural visualization, targeted biopsies, and pleurodesis if malignant effusion is confirmed.
- Bronchoscopy (if hemoptysis, bronchial obstruction, or lung mass is present).
This algorithm provides a framework for a rational and efficient approach to pleural fluid diagnosis, ensuring that appropriate investigations are performed to determine the underlying cause and guide optimal patient management.
Special Considerations in Pleural Effusions
Malignant Pleural Effusion
Malignant pleural effusion is a common complication of cancer, indicating advanced disease and portending a poor prognosis. Lung cancer is the most frequent primary malignancy associated with malignant pleural effusion, followed by breast cancer and lymphoma. Prognosis varies depending on the primary tumor type, with lung cancer having the poorest survival.
Diagnosis of malignant pleural effusion relies on cytological examination of pleural fluid. However, cytology may be negative in some cases, particularly in mesothelioma. In patients with known malignancy and pleural effusion with negative cytology, thoracoscopy with pleural biopsy should be considered to confirm or exclude pleural metastasis.
Management of malignant pleural effusion focuses on symptom palliation, primarily relief of dyspnea. Treatment options include:
- Therapeutic thoracentesis: For temporary symptom relief, but effusions frequently recur.
- Indwelling pleural catheter (IPC): Provides long-term drainage, effective for symptom control, and can be managed in the outpatient setting.
- Pleurodesis: Procedure to obliterate the pleural space, preventing fluid re-accumulation. Talc pleurodesis is considered the most effective agent. Pleurodesis can be performed chemically via chest tube or surgically via thoracoscopy. Pleurodesis is most effective when the lung is fully re-expanded after drainage.
The choice of treatment depends on the patient’s symptoms, performance status, tumor type, response to systemic therapy, and lung re-expansion capacity.
Parapneumonic Effusion and Empyema
Parapneumonic pleural effusion, effusion associated with pneumonia, is a common complication of bacterial pneumonia. Complicated parapneumonic effusions and empyema (pus in the pleural space) are associated with increased morbidity and mortality. Empyema incidence is rising, and nosocomial empyemas have a significantly higher mortality than community-acquired empyemas.
Diagnosis of empyema is based on pleural fluid analysis. Purulent pleural fluid is diagnostic. In non-purulent parapneumonic effusions, pleural fluid pH < 7.2, glucose < 60 mg/dL, and elevated LDH suggest a complicated effusion requiring drainage.
Management of complicated parapneumonic effusions and empyema involves:
- Antibiotics: Appropriate antibiotic therapy targeting the causative pathogens.
- Pleural drainage: Chest tube drainage is indicated for empyema and complicated parapneumonic effusions (pH < 7.2).
- Fibrinolytic therapy: May be considered to improve drainage in loculated effusions.
- Thoracoscopy (VATS): Early VATS is highly effective in managing empyema, facilitating debridement and drainage, and may improve outcomes compared to delayed intervention. Interdisciplinary management involving pulmonologists and thoracic surgeons is crucial.
Prompt diagnosis and drainage are critical in managing parapneumonic effusions and empyema to prevent complications and improve patient outcomes.
Hepatic Hydrothorax
Hepatic hydrothorax, pleural effusion in the setting of hepatic cirrhosis, is usually a transudate and indicates hepatic decompensation. It occurs in 4-10% of patients with advanced cirrhosis, typically on the right side, and is often associated with ascites.
Management of hepatic hydrothorax primarily focuses on treating the underlying ascites with sodium restriction and diuretics. Therapeutic thoracentesis may be necessary for symptomatic relief. In refractory cases or spontaneous bacterial empyema of hydrothorax (SBEM), transjugular intrahepatic portosystemic shunt (TIPS) or thoracoscopic interventions may be considered in select patients. Interdisciplinary management involving hepatologists and pulmonologists is essential.
Conclusion: Enhancing Pleural Fluid Diagnosis for Improved Patient Outcomes
Pleural fluid diagnosis is a critical component of managing patients with pleural effusion. A systematic approach encompassing clinical assessment, imaging, and comprehensive pleural fluid analysis is essential for accurate diagnosis and appropriate management. Pleural fluid analysis, guided by Light’s criteria and advanced biochemical, cytological, and microbiological tests, plays a central role in differentiating transudates from exudates and identifying specific etiologies, including infection and malignancy. Utilizing a structured diagnostic algorithm and considering special clinical scenarios, such as malignant pleural effusion, parapneumonic effusion/empyema, and hepatic hydrothorax, clinicians can optimize pleural fluid diagnosis, leading to improved patient outcomes through targeted and timely interventions.
References
(References should be added here, mirroring the original article’s references)