Introduction
Infectious diseases pose a significant global health challenge, and the ability to rapidly and accurately diagnose infections is paramount for effective patient care and public health management. Prompt diagnosis facilitates the prescription of appropriate treatments, guides epidemiological surveillance, and informs preventative measures. Traditionally, pathogen identification has relied on techniques such as microscopy, phenotypic profiling, antigen detection, and PCR-based genetic analysis. However, these methods often suffer from limitations in terms of time, cost, and the need for specialized laboratory infrastructure and skilled personnel. This restricts their accessibility, particularly in resource-limited settings. Recent advancements in diagnostic technologies, notably matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and point-of-care (POC) laboratories, offer disruptive solutions for rapid and cost-effective pathogen diagnosis, especially in regions like rural Senegal. These innovations, championed by institutions like the IHU-FMI, are transforming the landscape of clinical microbiology in tropical countries.
This article explores the application of MALDI-TOF MS and POC laboratories in Senegal, detailing their methodologies, advantages, and impact on pathogen diagnosis and healthcare delivery in rural communities.
The Transformative Power of MALDI-TOF MS for Pathogen Identification
MALDI-TOF MS represents a paradigm shift in microbial identification. This technique generates a unique mass spectrum for each microorganism by measuring the mass-to-charge ratio of ionized peptides within a sample. These spectra, acting as molecular fingerprints, can be derived from bacterial colonies or directly from complex clinical samples like blood. The power of MALDI-TOF MS lies in its ability to rapidly identify microorganisms by comparing the generated spectrum against extensive databases of reference spectra from well-characterized organisms. The IHU-FMI institute, a leader in this field, operates multiple MALDI-TOF mass spectrometers, driving innovation and application of this technology.
The MALDI-TOF MS workflow is remarkably streamlined and efficient compared to conventional methods, as illustrated in Fig. 1. Sample preparation is quick, and a technician can be trained in just a couple of days to perform routine bacterial identification (biotyping) using MALDI-TOF MS. The cost per sample analysis is significantly low, primarily involving reagents and consumables, making it an economically viable option for routine diagnostics. While the initial investment in a MALDI-TOF MS instrument and its maintenance constitutes the major cost, the high throughput – capable of processing tens of thousands of samples annually – drastically reduces the per-test cost. At IHU-FMI, the estimated consolidated cost per strain identification is remarkably low, highlighting the cost-effectiveness of this technology.
Because of its speed, reliability, and affordability, MALDI-TOF MS has been adapted for a wide range of applications beyond routine bacterial and yeast identification, as summarized in Table 1. These include the identification of Archaea and even giant viruses. Furthermore, MALDI-TOF MS can be utilized to monitor antibiotic resistance by detecting the degradation of antibiotics by bacteria. Recognizing the role of arthropods as vectors of numerous tropical diseases, researchers have successfully applied MALDI-TOF MS in entomology. It enables species-level identification of various arthropods, including ticks, mosquitoes, fleas, and Culicoides, at both adult and larval stages. This capability extends to the dual identification of arthropods and the vector-borne pathogens they carry, such as Plasmodium, filariae, Borrelia, and Rickettsia. MALDI-TOF MS can also determine the origin of blood meals in mosquitoes, providing valuable insights for vector-borne disease surveillance. Beyond infectious disease applications, MALDI-TOF MS is being explored for cell and tissue identification, including tumor characterization, and for traceability purposes, such as detecting microbial contamination in platelet concentrates and verifying animal food origin.
| MALDI-TOF-MS methods developed by IHU-Méditerranée Infection in collaboration with Marseille and Dakar research teams | Reference |
|---|---|
| Bacteria | Mycobacterium |
| Yersinia | [4] |
| Legionella | [6] |
| Bartonella | [5] |
| Antibiotic resistance | [10] |
| Archaea | [8] |
| Yeast | [7] |
| Giant viruses | [9] |
| Arthropods | Mosquitoes |
| Blood meal origin of mosquitoes | [31], [32] |
| Mosquito larvae | [25], [26] |
| Vectorized pathogens in mosquitoes | [27], [28] |
| Fleas | [16], [17] |
| Triatomines | [18] |
| Ticks and associated bacteria | [16], [19], [20], [21], [22], [29], [30] |
| Phlebotomines | [23] |
| Culicoides | [24] |
| Tissues or cells ID | Dental pulp |
| Cancer | [38] |
| Immune cell | [34], [35], [36], [37] |
| Traceability | Food origin |
| Bacterial contaminations of platelets | [39], [40] |
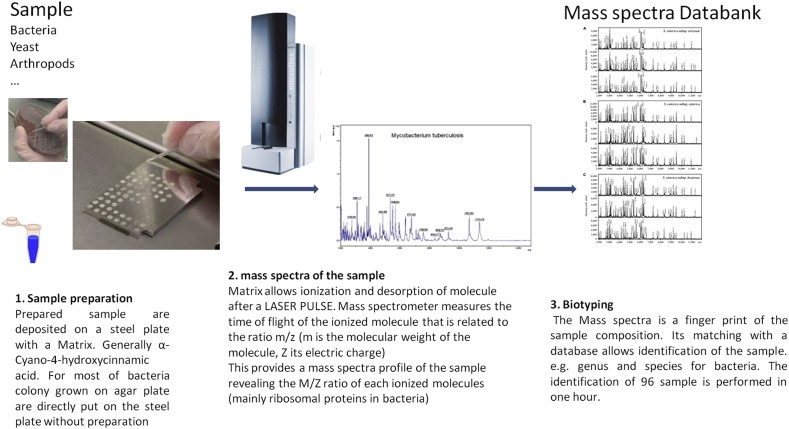
The accuracy of MALDI-TOF MS identification relies on sample quality and the comprehensiveness of reference spectral databases. The IHU-FMI has significantly contributed to database expansion through culturomics, a strategy to culture and characterize a wide diversity of bacteria, leading to the discovery of numerous novel species and the inclusion of their reference spectra in databases.
In a significant step towards expanding access to advanced diagnostics in Africa, IHU-FMI facilitated the installation of a MALDI-TOF MS system in the Principal Hospital of Dakar, Senegal, in 2012. This marked the first implementation of MALDI-TOF MS technology in Africa. This instrument is now routinely used in the clinical microbiology laboratory in Dakar, contributing to the discovery of new bacterial species and the development of specialized databases, such as for Culicoides identification. The successful implementation in Dakar underscores the robustness, reliability, and suitability of MALDI-TOF MS for deployment in African settings. Plans are underway to further expand MALDI-TOF MS access to Algeria and Mali.
Bringing Diagnostics Closer to Communities: Point-of-Care Laboratories in Rural Senegal
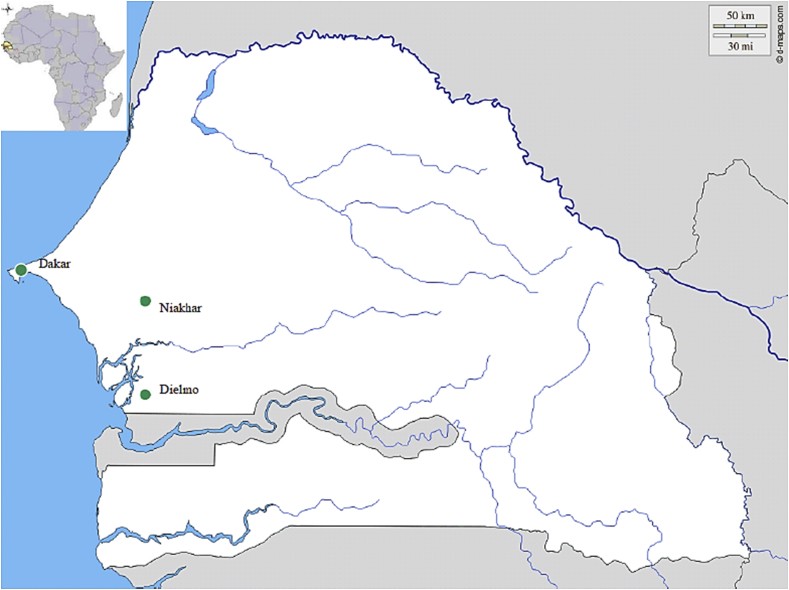
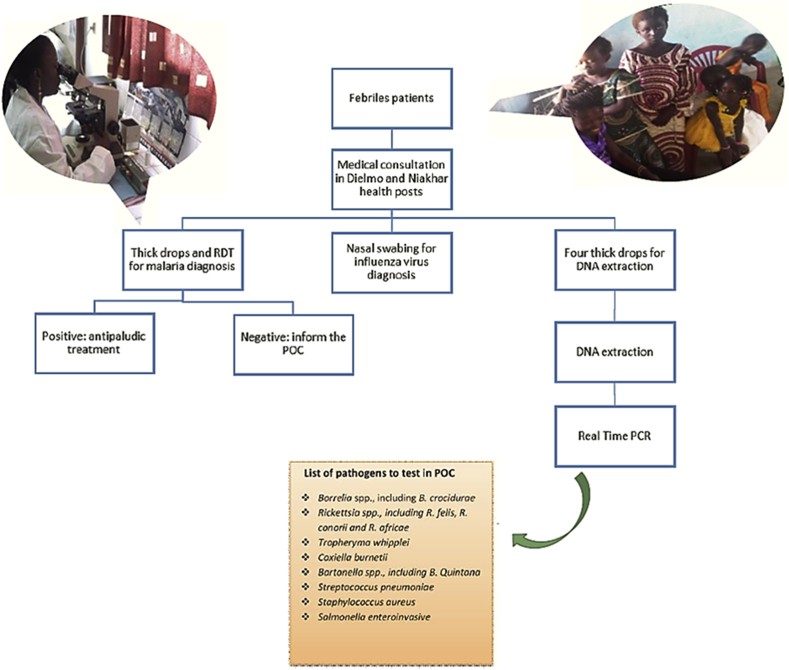
Point-of-care (POC) laboratories represent another crucial advancement in diagnostic accessibility, particularly for remote and underserved populations. These small, patient-proximal laboratories are designed to provide rapid diagnostic results, enabling timely clinical decisions. Recognizing the critical need for decentralized diagnostics in rural Senegal, two POC laboratories were established in the villages of Dielmo and Niakhar (Fig. 2). These POCs operate continuously, staffed by trained technicians proficient in performing rapid immunochromatographic tests, such as those for influenza virus detection, and real-time PCR assays for investigating bacteremia. Samples are collected from febrile patients seeking care at local health posts within the Dielmo and Niakhar study sites. DNA extraction is performed on-site using convenient blood extraction kits. To streamline operations in resource-limited settings, PCR reagents are prepared and lyophilized in Marseille, France, before being shipped to Senegal, ensuring ease of transport and storage. Upon arrival, the lyophilized mixes are simply reconstituted with RNAse-free water prior to PCR amplification. Diagnostic testing in these POCs employs a syndromic approach (Fig. 3), combining multiple tests to provide a comprehensive diagnosis based on the patient’s clinical presentation, thereby guiding appropriate medical management.
The significant volume of testing conducted in these rural Senegalese POCs – over 5,000 tests performed over eight years – unequivocally demonstrates the utility and acceptance of this approach for rapid, near-patient diagnosis. Furthermore, the data generated by these POC laboratories forms a crucial foundation for syndrome-based infectious disease surveillance in rural Senegal, enabling a more proactive and responsive public health system.
Conclusion
The successful implementation and operation of point-of-care laboratories and MALDI-TOF MS in Senegal exemplify the transformative potential of advanced technologies to strengthen healthcare systems in West Africa. By directly adopting cutting-edge diagnostic methods, Senegal is bypassing limitations associated with intermediate technologies and paving the way for improved health outcomes. These innovations not only enhance diagnostic capabilities but also promote equitable access to healthcare, particularly in rural communities, ultimately contributing to a healthier future for the region.
Acknowledgement
This study was supported by the Institut Hospitalo-Universitaire (IHU) Méditerranée Infection, the National Research Agency under the program « Investissements d’avenir », reference ANR-10-IAHU-03, the Région Provence Alpes Côte d’Azur and European funding FEDER PRIMI.
Conflict of interest
MD is cofounder and shareholder of Pocramé, a French startup involved in the POC business. The other authors declare that they have no conflict of interest.
