Introduction
Once considered an uncommon cause of secondary hypertension, primary aldosteronism (PA) is now recognized as a far more prevalent condition with significant implications for public health. The understanding of PA has evolved rapidly, driven by extensive translational research revealing it to be a common, underdiagnosed contributor to cardiovascular disease. Autonomous aldosterone secretion, the hallmark of PA, is not only a cause of hypertension but also independently increases cardiovascular morbidity. This review provides a practical, updated approach to the clinical Primary Aldosteronism Diagnosis and treatment strategies aimed at mitigating cardiovascular risks.
Pathophysiology of Primary Aldosteronism
The core feature of primary aldosteronism is the unregulated, excessive secretion of aldosterone from the adrenal glands. This secretion occurs independently of the normal regulatory mechanisms, which include angiotensin II, serum potassium levels, and adrenocorticotropic hormone (ACTH). Aldosterone exerts its effects by binding to the mineralocorticoid receptor (MR), primarily in the principal cells of the distal nephron within the kidneys. This binding initiates a cascade of events leading to sodium reabsorption through the epithelial sodium channel (ENaC) and a corresponding excretion of potassium and hydrogen ions.
This sodium retention drives water reabsorption, leading to volume expansion, increased glomerular filtration rate, and suppression of the renin-angiotensin-aldosterone system (RAAS), specifically renin and angiotensin II. The suppression of angiotensin II, a key regulator of sodium reabsorption in the proximal nephron, ironically enhances sodium delivery to the distal nephron. This amplifies the aldosterone-driven sodium reabsorption, further exacerbating volume expansion and potassium and acid excretion. These physiological changes are the basis for the classic clinical presentation of primary aldosteronism, characterized by hypertension, hypokalemia, and metabolic alkalosis.
Beyond the renal-MR pathway, autonomous aldosterone secretion also triggers pathological processes through extra-renal MR activation, particularly in the heart and blood vessels. The combination of fluid overload and excessive MR activation is thought to be a key mechanism behind the blood pressure-independent cardiovascular damage observed in primary aldosteronism. This highlights the importance of early and accurate primary aldosteronism diagnosis to prevent long-term complications.
Prevalence of Primary Aldosteronism
Historically, primary aldosteronism was thought to be a rare cause of hypertension. However, contemporary research indicates that it is a common condition, frequently missed in clinical practice. Estimating the true prevalence is challenging due to several factors. Firstly, robust prevalence studies require large, representative population samples, which have been lacking. Secondly, a universally accepted definition of primary aldosteronism remains elusive, contributing to variability in prevalence estimates across studies. The lack of a definitive gold standard, such as histopathology, further complicates prevalence determination. The flexible interpretation of what constitutes “autonomous aldosterone secretion” sufficient for a primary aldosteronism diagnosis has led to a wide range of reported prevalence rates.
Despite these challenges, primary aldosteronism is now recognized as the most common form of endocrine hypertension. A significant study by Monticone et al., involving 1,672 primary care hypertensive patients, rigorously screened for PA. Using stringent diagnostic criteria, they found a 6% prevalence of PA in the general hypertensive population. The prevalence increased with hypertension severity, reaching approximately 12% in patients with untreated blood pressure of 160–179/100–109 mmHg. Even in milder hypertension (140–159/80–99 mmHg), a 4% prevalence was observed. Among patients undergoing adrenal venous sampling (AVS) in this study, about one-third had unilateral disease, often due to an aldosterone-producing adenoma (APA), while two-thirds had bilateral disease, typically bilateral adrenal hyperplasia (BAH) or idiopathic hyperaldosteronism. These findings are consistent with prevalence rates reported in diverse populations globally.
It is crucial to acknowledge that variations in screening thresholds and confirmatory testing cut-offs can significantly impact prevalence estimates. Less stringent criteria might yield higher prevalence, potentially including milder forms of autonomous aldosterone secretion, while stricter criteria may underestimate the true burden by missing milder cases.
In populations with more severe hypertension, such as resistant hypertension, primary aldosteronism prevalence estimates are even higher, ranging from 12% to 20%. Emerging evidence suggests that even these higher estimates might be underestimates, as they may not capture milder forms of autonomous aldosterone secretion that do not meet current diagnostic thresholds for primary aldosteronism diagnosis.
Adverse Health Outcomes Associated with Untreated Primary Aldosteronism
The significant prevalence of primary aldosteronism is underscored by its associated clinical consequences, especially when diagnosis and treatment are delayed. Numerous studies have demonstrated that individuals with untreated primary aldosteronism face a significantly elevated risk of adverse health outcomes compared to those with essential hypertension, even when blood pressure levels are similar. These risks are largely independent of blood pressure and primarily affect cardiometabolic health.
Key cardiometabolic outcomes studied include myocardial infarction, heart failure, stroke, atrial fibrillation, diabetes, and metabolic syndrome. A comprehensive meta-analysis of multiple studies confirmed that the odds ratios for almost all clinically relevant cardiometabolic events were significantly higher in patients with untreated primary aldosteronism compared to those with essential hypertension (See Table 1). Furthermore, untreated primary aldosteronism is associated with increased risk of kidney disease, including glomerular hyperfiltration and albuminuria, and even increased mortality.
Table 1: Odds Ratios for Cardiovascular Outcomes in Untreated Primary Aldosteronism vs. Essential Hypertension
| Odds Ratio | 95% Confidence Interval | Outcome |
|---|---|---|
| 1.77 | 1.10–2.83 | Coronary artery disease (myocardial infarction or revascularization) |
| 2.58 | 1.93–3.45 | Stroke |
| 3.52 | 2.06–5.99 | Atrial Fibrillation |
| 2.05 | 1.11–3.78 | Heart Failure |
| 1.33 | 1.01–1.74 | Diabetes |
| 1.53 | 1.22–1.91 | Metabolic Syndrome |
Data from Monticone S, D’Ascenzo F, Moretti C, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50.
The substantial cardiometabolic risks associated with untreated primary aldosteronism emphasize the critical need for early primary aldosteronism diagnosis and targeted therapy to improve patient outcomes and reduce long-term morbidity and mortality.
Diagnostic Approach for Primary Aldosteronism
Given the significant cardiovascular risks associated with primary aldosteronism, early and accurate primary aldosteronism diagnosis is crucial. This allows for timely initiation of targeted therapy, which can significantly improve patient outcomes.
Who Should Be Screened for Primary Aldosteronism?
Current guidelines from the Endocrine Society recommend targeted screening for primary aldosteronism in patient populations with a higher prevalence of the condition (See Table 2). These indications are designed to identify individuals at increased risk and facilitate efficient case detection.
Table 2: Indications for Primary Aldosteronism Screening
| Indication |
|---|
| Blood pressure > 150/100 mmHg on three separate occasions |
| Resistant hypertension (blood pressure > 140/90 mmHg despite ≥3 antihypertensive medications including a diuretic) |
| Controlled hypertension with ≥4 antihypertensive medications |
| Hypertension with hypokalemia (spontaneous or diuretic-induced) |
| Hypertension with adrenal incidentaloma |
| Hypertension with sleep apnea |
| Hypertension with a family history of early-onset hypertension or stroke (age < 40 years) |
| Hypertension with a first-degree relative diagnosed with primary aldosteronism |
Alarmingly, studies indicate that only a small proportion of patients meeting these screening criteria are actually tested for primary aldosteronism. This highlights a critical gap in clinical practice and underscores the need for enhanced education regarding PA prevalence and the importance of primary aldosteronism diagnosis. The under-screening may be attributed to time and resource constraints in primary care settings, or a lack of awareness among non-hypertension specialists. It’s important to note that these guideline-based indications may primarily identify individuals with more severe and overt forms of PA, potentially missing milder cases where vascular damage may already be progressing. Recent research suggests that PA might be prevalent in less severe hypertension and even in normotensive individuals, groups not currently included in routine screening guidelines. Expanding screening criteria may be necessary to capture the full spectrum of primary aldosteronism.
How to Screen for Primary Aldosteronism
The aldosterone-to-renin ratio (ARR) is the most widely recommended initial screening test for primary aldosteronism diagnosis. ARR testing is convenient, typically requiring no special patient preparation, and can be performed in an outpatient setting. A commonly accepted positive screening threshold is an ARR > 30 ng/dL per ng/mL/h, combined with a serum aldosterone level > 15 ng/dL. However, it is crucial to consider the absolute aldosterone and renin values rather than solely relying on the ARR metric. The focus should be on identifying inappropriately elevated aldosterone levels in the context of suppressed renin, indicating autonomous aldosterone secretion. The clinical challenge lies in defining the lower limit of aldosterone elevation that still signifies clinically relevant autonomous secretion. Diagnostic cutoffs for a positive ARR screen vary due to the lack of a universally validated gold standard for primary aldosteronism diagnosis.
More lenient criteria may accept lower aldosterone levels (e.g., > 6 ng/dL or > 9 ng/dL) when accompanied by suppressed plasma renin activity (PRA) (e.g., < 1 ng/mL/h). Renin suppression or low renin is generally a biochemical prerequisite to support the diagnosis of autonomous aldosterone secretion. The determination of the aldosterone level considered a “positive screen” often depends on individual practice preferences, cost considerations, and available resources. Using stricter criteria (higher aldosterone and very low renin) may effectively identify overt cases but risk missing milder forms (false negatives). Conversely, more relaxed criteria (modest aldosterone and low renin) may detect a broader spectrum of cases, including milder ones, but could increase false positive screening results.
Historically, PRA has been the standard renin measurement, predominantly used in research. However, a global shift towards using direct renin concentration assays is underway. This transition necessitates a recalibration of diagnostic thresholds and reliable methods for comparing PRA and renin concentration values. Similarly, aldosterone assays are increasingly performed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) instead of radioimmunoassay. LC-MS/MS aldosterone measurements are generally lower than those from other assays, suggesting that new clinically relevant aldosterone level ranges and diagnostic thresholds will need to be established for primary aldosteronism diagnosis.
A frequent clinical question concerns the need to discontinue antihypertensive medications before ARR testing to avoid false negative results. Practices vary geographically and among clinicians. The primary medications of concern are those that raise renin, potentially lowering the ARR (e.g., mineralocorticoid receptor antagonists, ENaC inhibitors). If renin remains suppressed despite these medications, the renin, aldosterone, and ARR values remain interpretable, and testing while on these medications is reasonable. However, if renin is not suppressed in a patient taking these medications, a washout period (typically 4-6 weeks, although often shorter) may be necessary before repeat testing. During washout, antihypertensive agents that do not affect renin measurements (e.g., alpha-blockers, hydralazine) and potassium supplementation can be used to manage blood pressure and potassium levels.
While ACE inhibitors and ARBs can raise renin in normal physiology, their impact in primary aldosteronism, where renin is already suppressed, is usually minimal and unlikely to cause significant diagnostic misinterpretation. Beta-blockers can lower renin, potentially increasing the risk of false-positive ARR results by reducing the denominator. However, renin reduction by beta-blockers typically also lowers angiotensin II and aldosterone, making false-positive ARR results solely due to beta-blocker effect uncommon. Similarly, while calcium channel blockers and diuretics can influence the ARR, their effects are generally not substantial enough to dramatically alter diagnostic testing. Therefore, many experts recommend screening for primary aldosteronism diagnosis when suspected, regardless of current medications. Washout of MR antagonists or ENaC inhibitors should be considered if renin is not suppressed. Washout of other medications may be considered in cases of interpretive uncertainty, but should be done cautiously with careful monitoring due to potential difficulties in managing hypertension and hypokalemia in PA patients.
Confirmatory Testing for Primary Aldosteronism
Confirmation of positive screening results is often necessary for primary aldosteronism diagnosis. However, in patients with hypertension, hypokalemia, undetectable renin activity or levels, and significantly elevated serum aldosterone levels (e.g., ≥ 15 or 20 ng/dL), further dynamic testing may not be required, and the diagnosis can be considered confirmed. A recent study even showed that very high ARR values not only confirmed PA but also strongly predicted unilateral APAs.
When initial screening results are not definitively conclusive, dynamic confirmatory tests are recommended. These tests are essentially aldosterone suppression tests. However, significant variability exists in protocols, result interpretation, and diagnostic thresholds. The four main recommended confirmatory tests for primary aldosteronism diagnosis are:
- Oral Sodium Loading Test: Patients consume a high-sodium diet (approximately 4–6000 mg sodium) for 3–4 days, sometimes with added sodium chloride tablets. Potassium supplementation is often needed due to increased potassium excretion. On the final day, a 24-hour urine sample is collected. A 24-hour urine aldosterone excretion > 12 mcg, with urine sodium excretion > 200 mEq, is considered diagnostic of primary aldosteronism. Values > 10 mcg/24hrs are also strongly suggestive.
- Saline Infusion Test: Patients receive a 2-liter intravenous infusion of isotonic saline over 4 hours. Traditionally performed in a recumbent position, recent studies suggest that a seated position may increase sensitivity without compromising specificity. Blood samples for renin, aldosterone, cortisol, and potassium are drawn before and after infusion. Post-infusion serum aldosterone > 10 ng/dL is diagnostic of primary aldosteronism. A level > 6 ng/dL is diagnostic if the post-infusion cortisol level is lower than pre-infusion, ruling out ACTH effects.
- Fludrocortisone Suppression Test: Patients receive fludrocortisone 0.1 mg every 6 hours for 4 days, along with sodium and potassium supplementation. On day 4, 7 AM serum cortisol is measured. At 10 AM (seated position), serum aldosterone, PRA, and cortisol are measured. A serum aldosterone > 6 ng/dL with suppressed PRA confirms primary aldosteronism.
- Captopril Challenge Test: Patients receive 25–50 mg of captopril after sitting or standing for at least 1 hour. Serum aldosterone and PRA are measured at baseline and at 1 and 2 hours post-captopril, remaining seated throughout. In normal individuals, aldosterone will be suppressed, but in primary aldosteronism, aldosterone remains elevated, and PRA remains suppressed. Various diagnostic thresholds exist, including < 30% aldosterone suppression from baseline with persistent PRA suppression. An aldosterone-to-renin ratio >20 or >30 ng/dL per ng/mL/h, or failure to suppress aldosterone below 11 ng/dL, are also considered diagnostic.
Lateralization of Disease
After confirming the primary aldosteronism diagnosis, determining whether the disease is unilateral or bilateral is crucial for treatment planning. Cross-sectional imaging, such as CT scans, is recommended even for patients not considering surgery to rule out rare aldosterone-producing adrenocortical carcinoma (See Figure 1). However, imaging alone is not reliable for determining laterality and can be misleading. Non-functional adrenal incidentalomas are common, and PA can be present even without visible adrenal abnormalities on imaging. The PASO study demonstrated that adrenal venous sampling (AVS) significantly improved biochemical cure or improvement rates compared to CT-based localization. Therefore, AVS is generally recommended for localization in most PA patients who are surgical candidates. In younger patients and those with clear unilateral adenomas on CT and classic PA presentation, AVS may be selectively omitted.
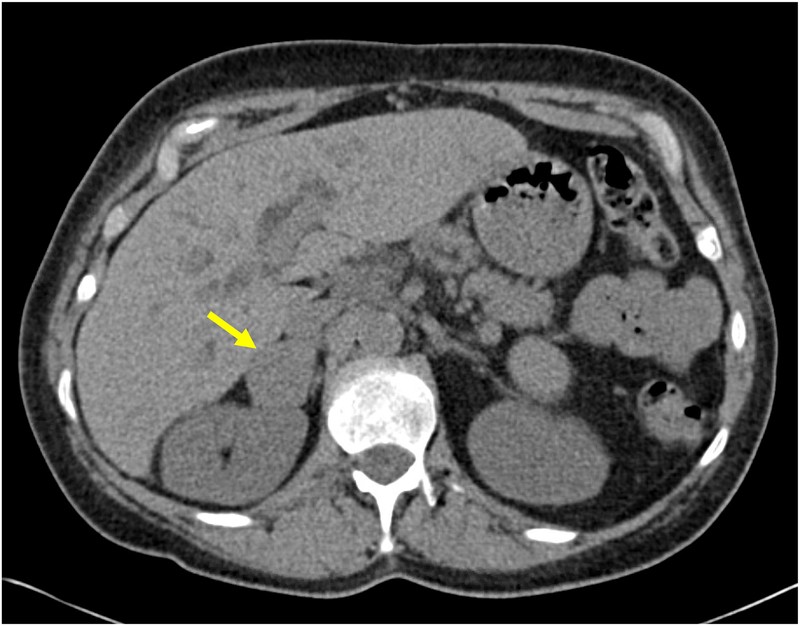
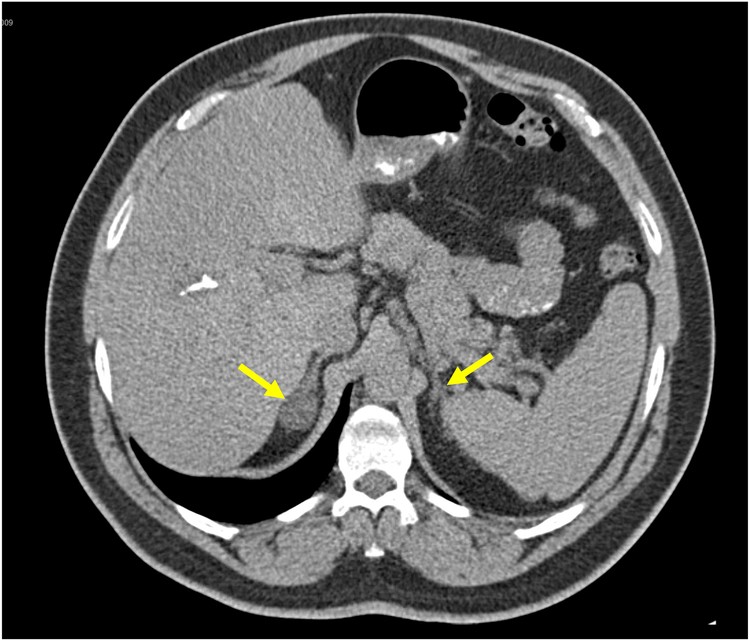
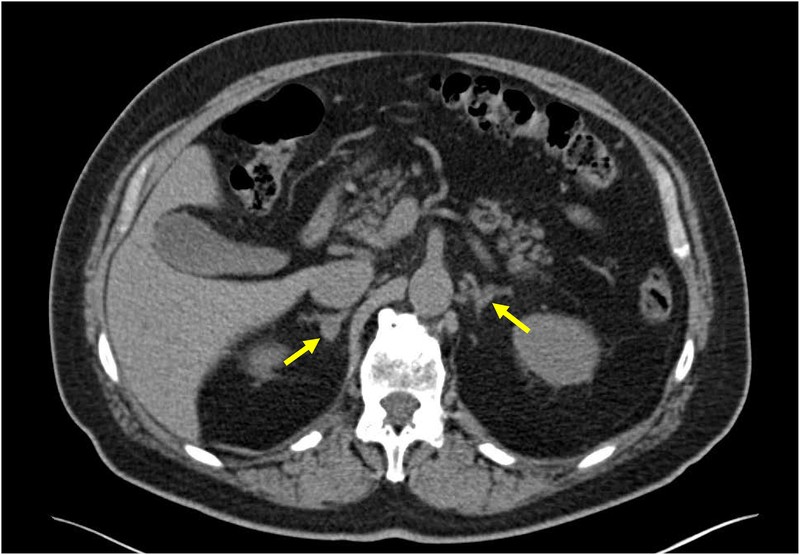
Figure 1: Adrenal CT Scans in Primary Aldosteronism
Figure 1A: A CT scan showing a 3.8 cm right adrenocortical carcinoma in a patient presenting with hypokalemia, hypertension, and confirmed primary aldosteronism. Alt text: CT scan of adrenal gland showing a large tumor indicative of adrenocortical carcinoma in a patient with primary aldosteronism.
Figure 1B: A CT scan showing a 2.1 cm right adrenocortical adenoma and a normal appearing left adrenal gland in a patient with confirmed primary aldosteronism. Adrenal venous sampling confirmed bilateral aldosterone production, and the patient was treated with mineralocorticoid receptor antagonists. Alt text: CT scan showing adrenal adenoma but AVS confirming bilateral aldosterone production in primary aldosteronism patient.
Figure 1C: A CT scan showing a 1.3 cm right adrenocortical adenoma and a normal appearing left adrenal gland in a patient with confirmed primary aldosteronism. Adrenal venous sampling confirmed unilateral aldosterone production from the right side, and the patient underwent a curative right adrenalectomy. Alt text: CT scan and AVS confirming unilateral aldosterone production from adrenal adenoma in primary aldosteronism patient.
The SPARTACUS trial, a randomized controlled trial, compared CT-based versus AVS-based localization and therapy. It concluded that both approaches yielded similar blood pressure control at one year. However, this study has limitations, including its short follow-up duration and potential misclassification, and its findings have not significantly altered clinical practice guidelines for primary aldosteronism diagnosis and management.
AVS is technically challenging and should be performed by experienced specialists. Optimal AVS methodology and protocols are subjects of ongoing debate and detailed discussion within the field.
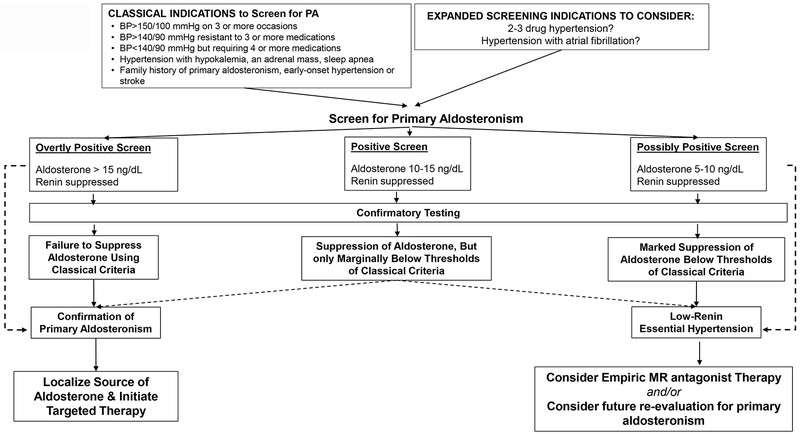
Proposed Diagnostic Algorithm for Primary Aldosteronism
Figure 2 presents a proposed diagnostic algorithm for primary aldosteronism diagnosis, acknowledging the condition’s spectrum of severity.
Figure 2: Proposed Diagnostic Approach for Primary Aldosteronism
Figure 2: Proposed Diagnostic Approach for Diagnosis of Primary Aldosteronism. Alt text: Diagnostic algorithm flowchart for primary aldosteronism diagnosis, starting with screening and progressing to confirmatory testing and lateralization.
Adapted from Vaidya A, Mulatero P, Baudrand R, Adler GK. The Expanding Spectrum of Primary Aldosteronism: Implications for Diagnosis, Pathogenesis, and Treatment. Endocr Rev. 2018;39(6):1057–88.
This algorithm begins with biochemical screening based on classical indications, as per Endocrine Society guidelines. Considering expanded screening criteria may improve detection rates. A positive screen suggests renin-independent aldosterone secretion, indicated by relatively high aldosterone levels with suppressed renin. If screening results are inconclusive, confirmatory testing is recommended. Failure or limited suppression of aldosterone during dynamic testing supports the primary aldosteronism diagnosis, while marked suppression may suggest low-renin essential hypertension. The diagnosis should be viewed as a spectrum, allowing for the identification of mild and non-classical cases.
Genetics of Primary Aldosteronism
Genetic testing is not routinely used in the clinical evaluation of primary aldosteronism, as inherited forms are rare. However, understanding the genetics of PA is important, particularly in specific clinical scenarios. Recent reviews provide detailed discussions of PA genetics. This section provides a concise overview of genetic aspects relevant to routine clinical practice in primary aldosteronism diagnosis.
Glucocorticoid-Remediable Aldosteronism (GRA)
Glucocorticoid-remediable aldosteronism (GRA), also known as Familial Hyperaldosteronism type I (FH-I), is a rare autosomal dominant form of PA caused by bilateral adrenal hyperplasia. GRA accounts for less than 1% of PA cases. It results from a genetic mutation causing a fusion of the promoter region of the CYP11B1 (11β-hydroxylase) gene and the coding sequence of the CYP11B2 (aldosterone synthase) gene. This leads to ACTH-driven aldosterone secretion. GRA typically presents in younger patients with a strong family history of PA, early-onset hypertension, or hemorrhagic stroke at a young age. Hypokalemia may be less common in GRA due to the circadian rhythm of ACTH secretion. Clinical suspicion of GRA should be confirmed by genetic testing. If genetic testing is unavailable, dexamethasone suppression testing can be used as a second-line alternative. Dexamethasone (1 mg twice daily for three days) will suppress aldosterone in GRA.
Familial Hyperaldosteronism (FH) Types II–IV
Historically, familial PA cases excluding GRA were classified as FH-II. FH-II is still considered the most common familial form, typically inherited in an autosomal dominant pattern, and clinically similar to sporadic PA. The genetic basis of FH-II was largely unknown until recently. Linkage analyses mapped FH-II loci to chromosome 7p22. Recent studies have identified mutations in the CLCN2 chloride channel as a cause of inheritable PA, potentially accounting for some FH-II cases and prompting a reclassification of certain FH-II cases. FH-III, extremely rare, is caused by mutations in the KCNJ5 potassium channel and presents with severe childhood hypertension and PA. FH-IV, also exceedingly rare, results from mutations in the CACNA1H T-type voltage-gated calcium channel. The FH-IV phenotype can include neurocognitive disorders, epilepsy, and autism. De novo mutations in the L-type voltage-gated calcium channel gene CACNA1D can cause PA and are linked to childhood seizures and neurologic abnormalities (PASNA syndrome).
Genetics and Pathogenesis of Aldosterone-Producing Adenomas
While inherited forms of PA remain rare, it is now evident that most aldosterone-producing adenomas (APAs) harbor somatic pathogenic mutations leading to autonomous aldosterone secretion. A recent study found somatic mutations in 88% of APAs studied. The most frequent mutations were in KCNJ5 (43%), CACNA1D (21%), and ATP1A1 (17%). KCNJ5 mutations are more common in women, younger patients, associated with more severe PA phenotypes, and higher rates of cure after adrenalectomy. These somatic mutations affect voltage-gated calcium channels in zona glomerulosa cells, either directly or indirectly. Whether PA driven by these mutations is particularly responsive to calcium channel blockers remains unclear. Currently, mutation testing is not part of standard clinical practice for primary aldosteronism diagnosis, and the clinical implications of this genetic understanding are still evolving.
Aldosterone-Producing Cell Clusters
Non-neoplastic clusters of autonomous aldosterone-secreting cells, termed aldosterone-producing cell clusters (APCCs), have been identified in adrenal glands from individuals with and without PA in post-mortem studies. APCCs express CYP11B2, frequently harbor PA-related somatic mutations, are found in both normotensive and hypertensive individuals, and increase in prevalence with age. APCCs are hypothesized to contribute to BAH (idiopathic hyperaldosteronism), age-related autonomous aldosteronism, and potentially represent precursor lesions to neoplastic PA.
Management of Primary Aldosteronism
Management strategies for primary aldosteronism aim to normalize aldosterone levels and mitigate the associated cardiovascular risks. Treatment approaches include lifestyle modifications, pharmacological therapy, and surgical intervention.
Dietary Sodium Restriction
Similar to essential hypertension management, dietary sodium restriction is generally recommended for patients with primary aldosteronism. Reduced sodium intake can lead to volume contraction, increasing renin and angiotensin II levels. This rise in angiotensin II reduces distal sodium delivery, counteracting the effects of aldosterone-mediated sodium reabsorption. However, sodium restriction alone is typically insufficient to adequately manage PA and reduce long-term adverse health outcomes in most patients. Studies have shown that even with intense sodium restriction, achieving sustained normalization of ARR and adequate blood pressure control is challenging for most individuals in Western populations with typical sodium intakes.
Mineralocorticoid Receptor Antagonists
Lifelong mineralocorticoid receptor (MR) antagonist therapy is recommended for patients with bilateral primary aldosteronism and those with unilateral PA who are not surgical candidates. Spironolactone and eplerenone are the most commonly used MR antagonists. Spironolactone is more potent but carries a higher risk of gynecomastia in men, potentially affecting adherence. MR antagonists reduce ENaC-mediated sodium reabsorption, volume expansion, and potassium and hydrogen ion excretion. They effectively lower blood pressure and improve potassium balance in most patients with PA.
Despite the recommendation for MR antagonist therapy, questions remain about their effectiveness in reducing long-term adverse health outcomes compared to essential hypertension, and optimal dosing strategies. Recent cohort studies suggest that patients with PA treated with MR antagonists may still have worse long-term outcomes compared to essential hypertension. A large cohort study comparing cardiometabolic outcomes in PA patients on MR antagonists to age-matched essential hypertension patients with similar blood pressure control found that PA patients had a twofold higher risk of myocardial infarction, heart failure hospitalization, and stroke, despite MR antagonist treatment and similar blood pressure control. PA patients also had significantly higher risks of atrial fibrillation, diabetes mellitus, chronic kidney disease, and death.
Importantly, this excess cardiovascular risk was primarily observed in patients whose renin remained suppressed despite MR antagonist therapy. Patients who achieved a substantial increase in renin (≥1.0 ng/mL/h) with MR antagonist treatment, often requiring higher doses, had a lower risk of cardiovascular events and mortality, similar to that of essential hypertension patients. These findings, along with previous studies, highlight that much of the excess cardiovascular risk in primary aldosteronism is independent of blood pressure control. Blood pressure alone may not be a sufficient marker of treatment efficacy. In contrast, a rise in renin, indicating effective MR blockade and volume contraction, may be a clinically useful biomarker for MR antagonist adequacy. Achieving a significant renin increase often requires more aggressive MR antagonist dosing, which may be limited by spironolactone’s anti-androgenic effects or the risk of hyperkalemia, especially in patients with kidney disease. Combining MR antagonists with sodium restriction to normalize blood pressure and potassium and increase renin activity into the detectable range may be the ideal treatment approach, when achievable.
Surgical Adrenalectomy
Surgical adrenalectomy is the preferred treatment for patients with unilateral primary aldosteronism who are healthy enough for surgery. Laparoscopic or retroperitoneoscopic adrenalectomy is now the standard approach, resulting in lower complication rates and shorter hospital stays. Multiple studies have demonstrated high success rates in curing PA with surgery, evidenced by resolution of hypokalemia, improved or resolved hypertension, and biochemical cure. Direct comparisons between surgical adrenalectomy and MR antagonist therapy are limited due to inherent biases in comparing unilateral versus bilateral disease. However, studies attempting to control for these differences suggest that surgical adrenalectomy is associated with improved long-term cardiovascular and renal outcomes, and reduced mortality, compared to MR antagonist therapy. Therefore, surgical adrenalectomy is strongly recommended for suitable patients with unilateral primary aldosteronism.
An important unanswered question is whether unilateral adrenalectomy should be considered in selected cases of bilateral primary aldosteronism to attenuate disease severity, even if not curative. Given the potentially inferior outcomes of MR antagonist therapy compared to both surgery for unilateral PA and essential hypertension, unilateral adrenalectomy in bilateral PA, particularly when medical management is challenging due to medication side effects or uncontrolled disease, could reduce the burden of autonomous aldosterone secretion. However, data on this approach are limited, and the decision should be individualized based on clinical judgment.
Conclusion
Primary aldosteronism is a common and often underdiagnosed cause of hypertension, associated with significant morbidity and mortality beyond its effect on blood pressure. This review has summarized current knowledge and provided a practical diagnostic and treatment approach based on recent data. Future research, particularly prospective and interventional studies, is needed to improve the early recognition of PA, including milder forms, and to develop personalized therapies to optimize outcomes for patients with primary aldosteronism diagnosis.
Footnotes
Publisher’s Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
(References are identical to the original article and thus not repeated here for brevity)