Ptosis, clinically termed blepharoptosis, is characterized by the drooping of the eyelid, specifically a reduction in the palpebral aperture. While commonly associated with the upper eyelid, ptosis can also manifest in the lower eyelid as inverse or reverse ptosis. Recognizing ptosis is crucial as it can be an indicator of various underlying conditions, some of which are serious neurological disorders such as Horner syndrome, third cranial nerve palsy, or myasthenia gravis. A meticulous approach involving detailed patient history, review of past photographs, and precise measurements of visual function is essential to accurately characterize ptosis, determine the necessity for further investigation, assess urgency, and guide appropriate treatment strategies.
Understanding the Etiology of Ptosis
Acquired ptosis arises from a spectrum of causes, ranging from age-related physiological changes to potentially life-threatening medical emergencies. Broadly, the etiologies of acquired ptosis are categorized into aponeurotic, traumatic, mechanical, myogenic, and neurogenic types. Given that ptosis can be a presenting sign of significant underlying neurological conditions (as detailed in the Table below), it is paramount for clinicians to detect even subtle degrees of eyelid drooping, irrespective of symptom presentation. The severity of ptosis does not reliably correlate with the underlying cause, underscoring the need for thorough evaluation in all cases.
Categories of Acquired Ptosis
Aponeurotic Ptosis: Also known as senile or involutional ptosis, this is the most prevalent form of acquired ptosis, primarily linked to aging. It results from the stretching, thinning, or disinsertion of the levator aponeurosis, the tendon-like structure of the levator palpebrae superioris muscle.
Mechanical Ptosis: This type occurs when the eyelid’s weight increases to the point where the muscles responsible for elevation are unable to maintain the normal eyelid position. Common causes include eyelid tumors, cysts, or excessive dermatochalasis (redundant eyelid skin).
Myogenic Ptosis: In contrast to aponeurotic ptosis, myogenic ptosis stems from intrinsic dysfunction of the levator muscle itself. This impairs the muscle’s ability to effectively elevate the eyelid. Importantly, Müller’s muscle function is typically unaffected in this type of ptosis.
Neurogenic Ptosis: This category arises from neurological impairment affecting the nerves controlling eyelid elevation. The oculomotor nerve (third cranial nerve), sympathetic nerves, or central nervous system pathways can be involved.
Traumatic Ptosis: Ptosis resulting from trauma can encompass elements of aponeurotic, mechanical, myogenic, and neurogenic mechanisms, depending on the nature and extent of the injury.
Source: Morris CL, Chesnutt DA. Acquired ptosis: evaluation and management. American Academy of Ophthalmology. February 2005. www.aao.org/eyenet/article/acquired-ptosis-evaluation-management. Accessed March 16, 2021.
Essential Eyelid Measurements for Ptosis Diagnosis
While asymmetry in eyelid position is a suggestive sign of ptosis, it is not always present; ptosis can be unilateral or bilateral. Precise eyelid measurements and functional assessments are indispensable for an accurate diagnosis of ptosis. These evaluations are readily incorporated into a standard comprehensive eye examination. The identification and quantification of ptosis typically involve measuring the palpebral aperture and the marginal reflex distance-1 (MRD-1).
Figure 1. Eyelid measurements crucial for identifying ptosis. (A) Demonstrates corneal light reflex and upper and lower eyelid margins. (B) Palpebral aperture measurement: distance from central upper to lower eyelid margin. (C) Marginal reflex distance-1 (MRD-1) measurement: distance from central upper eyelid margin to corneal light reflex.
The palpebral aperture is defined as the vertical distance between the upper and lower eyelid margins when the patient is looking straight ahead. MRD-1 is the distance from the upper eyelid margin to the corneal light reflex, generated by directing a penlight at the cornea. To ensure measurement accuracy, it’s crucial to minimize frontalis muscle action, which can artificially elevate the upper eyelid. This is achieved by gently applying pressure with the palm of your hand to the patient’s forehead, preventing brow elevation (Figure 2). During both palpebral aperture and MRD-1 measurements, the patient should maintain a straight gaze, without any head or chin tilting.
Figure 2. Correct technique for frontalis muscle immobilization during eyelid measurements to diagnose ptosis.
Further valuable eyelid measurements include assessing the eyelid crease and levator muscle function. The eyelid crease represents the point of insertion of the levator aponeurosis into the upper eyelid. Its measurement, the distance from the upper eyelid margin to the crease while the patient looks down, can provide insights into aponeurotic ptosis. Levator disinsertion leads to a higher eyelid crease or an increased distance from the eyelid margin. Levator function, reflecting the excursion of the upper eyelid, is quantified by measuring the eyelid margin movement from downgaze to upgaze. Normal levator function is typically greater than 10mm.
Anatomical Considerations in Ptosis
A thorough understanding of eyelid anatomy is essential for comprehending the mechanisms of ptosis. The primary muscles involved in upper eyelid elevation are the levator palpebrae superioris and the superior tarsal muscle (Müller’s muscle).
Levator Palpebrae Superioris: This is the main muscle responsible for upper eyelid elevation, innervated by the oculomotor nerve (cranial nerve III). Dysfunction at any point along the levator pathway – muscle itself, aponeurotic insertion, third cranial nerve, or neuromuscular junction (as in myasthenia gravis) – can result in ptosis. Damage can lead to varying degrees of ptosis, from subtle drooping to complete eyelid closure.
Superior Tarsal Muscle (Müller’s Muscle): This muscle, originating from the undersurface of the levator, contributes to a smaller degree of upper eyelid elevation. Innervated by the sympathetic autonomic nervous system, its dysfunction leads to only mild ptosis.
Inferior Tarsal Muscle: Analogous to Müller’s muscle in the upper eyelid, the inferior tarsal muscle assists in lower eyelid depression (opening). Also sympathetically innervated, its dysfunction contributes to reverse ptosis of the lower eyelid, often seen in conjunction with upper eyelid ptosis in Horner syndrome.
Ptosis Differential Diagnosis: Key Considerations
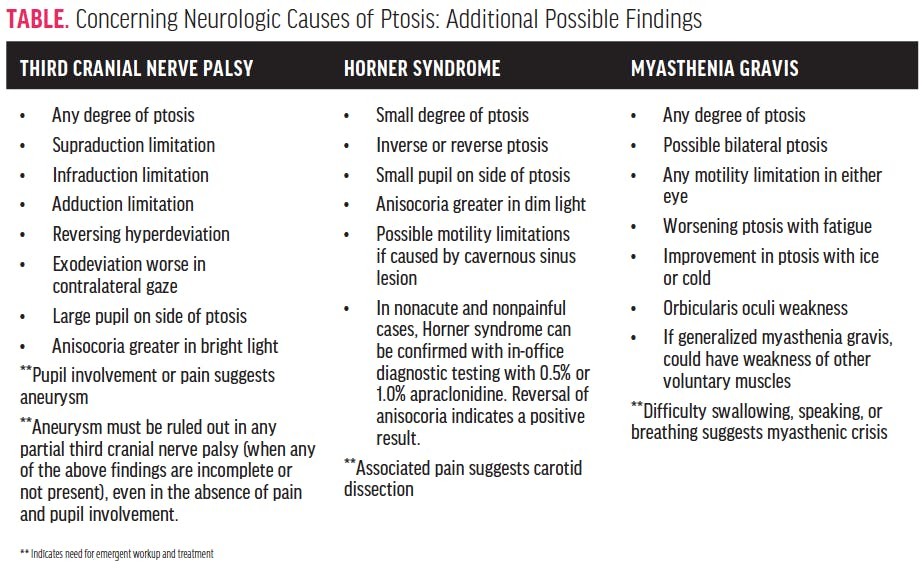
The differential diagnosis of ptosis is broad and necessitates careful consideration of various conditions. Key diagnostic considerations include Horner syndrome, third cranial nerve palsy, myasthenia gravis, and pseudoptosis.
Horner Syndrome: This syndrome arises from disruption of the sympathetic pathway innervating the eye and surrounding structures. Characterized by a triad of findings: miosis (pupillary constriction), anhidrosis (decreased sweating on the affected side of the face), and ptosis. The ptosis in Horner syndrome is typically mild (due to Müller’s muscle involvement). Crucially, anisocoria (unequal pupil size) is more pronounced in dim illumination because the sympathetic pathway is critical for pupillary dilation.
In cases of suspected Horner syndrome, particularly non-acute and non-painful presentations, pharmacological testing with apraclonidine eye drops (0.5% or 1.0%) is diagnostic. A reversal of anisocoria, where the smaller pupil (affected side) becomes larger than the contralateral pupil within an hour of apraclonidine instillation, confirms Horner syndrome. However, in acute and/or painful Horner syndrome, immediate referral to the emergency department for neuroimaging (CT or MR angiography) is mandatory to rule out carotid artery dissection, a potentially life-threatening cause.
Third Cranial Nerve Palsy: Ptosis is a prominent feature of third cranial nerve palsy, which can be caused by various etiologies, including compression from a posterior communicating artery aneurysm – a neurosurgical emergency. In addition to ptosis (due to levator palpebrae superioris palsy), third nerve palsy typically presents with other oculomotor deficits: limitation of eye movements (supraduction, infraduction, adduction), resulting in strabismus (exotropia and hypotropia). Pupillary involvement is critical; pupillary dilation (mydriasis) or anisocoria greater in bright light suggests compression of parasympathetic fibers, often by an aneurysm, demanding urgent neuroimaging.
Myasthenia Gravis: This autoimmune neuromuscular disorder can cause fluctuating and fatigable weakness of eyelid muscles, leading to ptosis. Ocular myasthenia gravis primarily affects the eye muscles, commonly causing ptosis and diplopia (double vision). Unlike Horner syndrome and third nerve palsy, myasthenia gravis does not cause pupillary abnormalities. Other suggestive signs include orbicularis oculi muscle weakness and positive fatigue tests (worsening ptosis with sustained upgaze) and ice pack test (improvement of ptosis after applying ice to the eyelid). While often requiring outpatient neurology referral and workup, myasthenic crisis, a severe exacerbation, is a medical emergency.
Pseudoptosis: Eyelid asymmetry can sometimes mimic true ptosis but is not due to eyelid muscle weakness. Pseudoptosis can result from enophthalmos (recession of the eyeball within the orbit) in one eye, creating the illusion of ptosis in that eye. Conversely, proptosis (protrusion of the eyeball) and eyelid retraction in the contralateral eye can also give the appearance of ptosis in the other eye. Dermatochalasis, or excess upper eyelid skin, is another frequent cause of pseudoptosis, where the redundant skin folds over the eyelid margin, mimicking drooping. Exophthalmometry measurements can help differentiate pseudoptosis from true ptosis by assessing for globe position asymmetry.
Management Strategies for Ptosis
A comprehensive evaluation, including detailed history, review of old photographs, and careful assessment of visual function, is crucial for characterizing ptosis, guiding further investigations, determining urgency, and planning treatment. Once serious underlying etiologies are ruled out, management focuses on addressing the ptosis itself. While surgical intervention has been the traditional mainstay of ptosis correction, a novel non-surgical option has emerged: oxymetazoline hydrochloride ophthalmic solution 0.1% (Upneeq). This alpha-adrenergic agonist, administered once daily, elevates the upper eyelid by stimulating Müller’s muscle, thereby improving the superior visual field.
Conclusion: Proactive Ptosis Assessment
Eye care providers should routinely assess patients for ptosis, even in the absence of symptoms. Incorporating eyelid assessment into routine comprehensive eye exams can significantly benefit patients by facilitating early detection of underlying conditions and improving their quality of life, both functionally and cosmetically. Early diagnosis and appropriate management of ptosis can have a profound positive impact on patient outcomes.
References:
-
- Latting MW, Huggins AB, Marx DP, Giacometti JN. Clinical evaluation of blepharoptosis: distinguishing age-related ptosis from masquerade conditions. Semin Plast Surg. 2017;31(1):5-16.
-
- Bartley GB, Frueh BR, Holds JB, Linberg JV, Patel BC, Hawes MJ. Lower eyelid reverse ptosis repair. Ophthalmic Plast Reconstr Surg. 2002;18(1):79-83.
-
- Zoumalan CI, Lisman RD. Evaluation and management of unilateral ptosis and avoiding contralateral ptosis. Aesthet Surg J. 2010;30(3):320-328.
-
- Kakizaki H, Malhotra R, Selva D. Upper eyelid anatomy: an update. Ann Plast Surg. 2009;63(3):336-343.
-
- Kakizaki H, Malhotra R, Madge SN, Selva D. Lower eyelid anatomy: an update. Ann Plast Surg. 2009;63(3):344-351.
-
- Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain. 2015;7:35-46.
-
- Koc F, Kavuncu S, Kansu T, et al. The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. Br J Ophthalmol. 2005;89(11):1442-1444.
-
- Davagnanam I, Fraser CL, Miszkiel K, Daniel CS, Plant GT. Adult Horner’s syndrome: a combined clinical, pharmacological, and imaging algorithm. Eye (Lond). 2013;27(3):291-298.
-
- Fang C, Leavitt JA, Hodge DO, Holmes JM, Mohney BG, Chen JJ. Incidence and etiologies of acquired third nerve palsy using a population-based method. JAMA Ophthalmol. 2017;135(1):23-28.
-
- Tamhankar MA, Biousse V, Ying GS, et al. Isolated third, fourth, and sixth cranial nerve palsies from presumed microvascular versus other causes: a prospective study. Ophthalmology. 2013;120(11):2264-2269.
-
- Jayam Trouth A, Dabi A, Solieman N, et al. Myasthenia gravis: a review. Autoimmune Dis. 2012;2012:874680.
-
- Sanders DB, Howard JF Jr, Johns TR. Single-fiber electromyography in myasthenia gravis. Neurology. 1979;29(1):68-76.
-
- Nair AG, Patil-Chhablani P, Venkatramani DV, Gandhi RA. Ocular myasthenia gravis: a review. Indian J Ophthalmol. 2014;62(10):985-991.
-
- Slonim CB, Foster S, Jaros M, et al. Association of oxymetazoline hydrochloride, 0.1%, solution administration with visual field in acquired ptosis: a pooled analysis of 2 randomized clinical trials. JAMA Ophthalmol. 2020;138(11):1168-1175.