Introduction
Lumbar radiculopathy represents a prevalent condition frequently encountered in clinical settings, particularly by spine surgeons. Epidemiological studies suggest that it affects approximately 3% to 5% of the general population, with no significant gender predilection, although some studies indicate a slight male predominance overall and higher female risk in specific demographics like those in physically demanding military roles. The incidence of lumbar radiculopathy rises with age, primarily due to degenerative changes within the spinal column. Symptom onset is typically observed in middle age, commonly in the 40s for men and the 50s and 60s for women [1, 2]. Degenerative spondyloarthropathies stand out as the primary underlying cause [1]. Patients often report back pain concurrently with radicular symptoms. Radiculopathy is characterized by pain radiating into the legs, frequently described as electric, burning, or sharp. The fundamental etiology involves irritation of a nerve root, which can occur at any point along its course but is most commonly due to compression. In the lumbar spine, this compression can happen within the thecal sac, in the lateral recess as the nerve root exits, within the neural foramina, or even distal to the foramina. Contributing factors to this compression include disc bulges or herniations, facet or ligamentous hypertrophy, spondylolisthesis, and, less frequently, neoplastic or infectious processes. Accurate Radiculopathy Diagnosis, crucial for effective management, begins with a comprehensive physical examination.
Review of Radiculopathy Diagnosis Methods
The cornerstone of radiculopathy diagnosis is a thorough clinical evaluation. This starts with detailed history taking and a comprehensive physical examination. The physical exam should systematically assess motor strength using manual muscle testing, sensory function via dermatomal testing, deep tendon reflexes, and provocative maneuvers like Lasegue’s sign, also known as the straight leg raise test [4]. To perform Lasegue’s sign, the patient is positioned supine with the knee extended and ankle dorsiflexed. The examiner then passively raises the patient’s leg towards 90 degrees while flexing the cervical spine. Reproduction of radicular pain during this maneuver is considered a positive Lasegue’s sign, indicating nerve root irritation due to stretching.
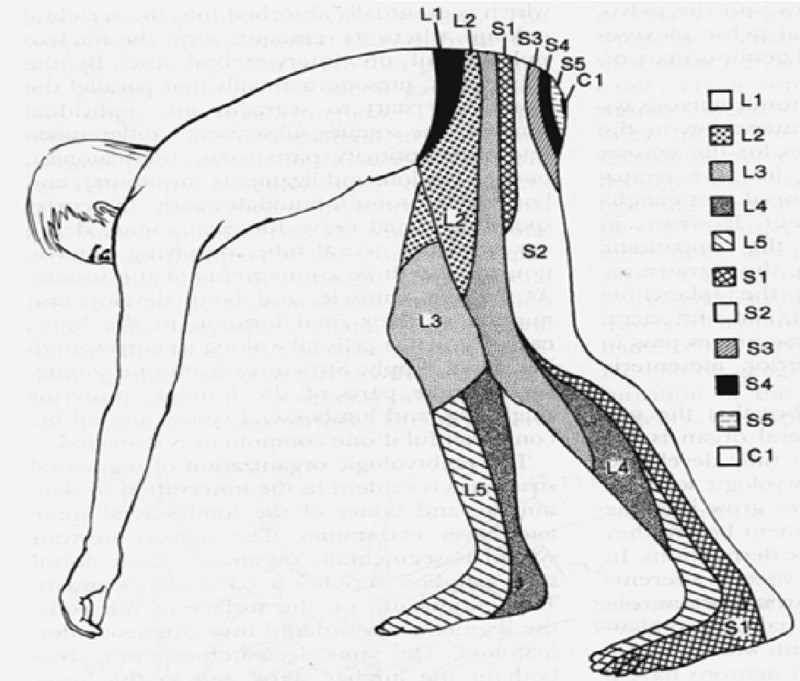
Sensory deficits in radiculopathy, particularly when caused by nerve root compression, typically follow a dermatomal distribution (Figure 1). Dermatomal maps are essential tools in radiculopathy diagnosis, illustrating the areas of skin innervated by specific nerve roots. Similarly, motor weakness may present in a myotomal pattern (Table 1), reflecting the muscles primarily innervated by a particular nerve root. The patterns of pain, sensory loss, and motor weakness identified during the physical examination are vital in guiding the diagnostic process. These findings help clinicians localize the affected nerve root level and direct further investigations, such as MRI and electrodiagnostic studies, to the relevant spinal region.
Figure 1. Dermatomes of the Lumbosacral Region.
Anatomical dermatome chart illustrating sensory distribution in the lumbosacral region, crucial for radiculopathy diagnosis and neurological assessments.
Table 1. Lumbosacral Myotomes and Associated Muscle Function.
| Spinal Nerve | Myotome |
|---|---|
| L2 | Hip Flexion (Iliopsoas) |
| L3 | Knee Extension |
| L4 | Ankle Dorsiflexion (Tibialis Anterior) |
| L5 | Ankle Eversion (Peroneus Longus and Brevis), Great Toe Extension (Extensor Hallucis Longus) |
| S1 | Plantar Flexion (Gastrocnemius, Soleus) |
Following the clinical assessment, diagnostic imaging plays a critical role in confirming the radiculopathy diagnosis and identifying the underlying pathology. Magnetic Resonance Imaging (MRI) of the lumbar spine, without contrast, is considered the gold standard imaging modality for evaluating radiculopathy. MRI excels at visualizing soft tissues, clearly demonstrating nerve root compression, disc herniations, and other structural abnormalities (Figure 2). While non-contrast MRI is typically sufficient, contrast-enhanced MRI may be indicated in specific scenarios, such as suspected tumors, infections, or in patients with prior spinal surgery to better delineate scar tissue from recurrent disc herniation.
In situations where MRI is contraindicated or unavailable, Computed Tomography (CT) myelography serves as a viable alternative. CT myelography involves injecting a contrast agent into the spinal canal followed by CT scanning, which can effectively visualize the spinal cord and nerve roots, identifying areas of compression.
Figure 2. Lumbar Spine MRI in Radiculopathy Diagnosis.
A. Sagittal T2-weighted MRI of the lumbar spine without contrast showing a significant L5/S1 paracentral disc protrusion. B. Axial T2-weighted MRI at the L5/S1 level demonstrating compression of the right S1 nerve root, indicative of right S1 radiculopathy.
Clinical Pearl:
It is paramount to correlate MRI findings with the patient’s clinical presentation. A frequent diagnostic pitfall is overlooking far lateral disc herniations if they are not specifically sought. MRI, being a triplanar imaging technique, requires careful review of axial, sagittal, and coronal sequences. Sagittal views are particularly useful for identifying foraminal disc herniations, while coronal sequences provide excellent visualization of nerve roots and the foraminal and extraforaminal regions where far lateral disc herniations commonly occur. A meticulous review across all planes is crucial for accurate radiculopathy diagnosis.
Electrodiagnostic testing, including Electromyography (EMG) and Nerve Conduction Velocity (NCV) studies, can be valuable when there is discordance between clinical findings and imaging results. These tests help differentiate radiculopathy from peripheral neuropathies or other conditions mimicking nerve root compression. EMG assesses the electrical activity of muscles, detecting abnormalities indicative of nerve damage, while NCV studies measure the speed of electrical signal transmission along nerves, identifying nerve dysfunction. Somatosensory Evoked Potentials (SSEPs) are another electrodiagnostic modality that can be used to evaluate the sensory pathways from the periphery to the brain.
However, it is essential to recognize the limitations of EMG and NCV studies. The accuracy of these tests can be influenced by patient factors such as cooperation level (which may be limited by pain), room temperature, fluid and electrolyte balance, pre-existing conditions like diabetes or thyroid disease (which can cause peripheral neuropathy), medications such as statins (which can induce myopathy), movement disorders, prior surgeries (e.g., laminectomy potentially causing false positives in paraspinal muscles), body habitus (obesity may hinder needle insertion), congenital anatomical variations, and the subjective interpretation of the data by the examiner [5]. In certain cases, diagnostic nerve root blocks, involving the injection of local anesthetic around a specific nerve root, can aid in localizing the symptomatic level and confirming the radiculopathy diagnosis [6].
Treatment Approaches Following Radiculopathy Diagnosis
Upon confirming a radiculopathy diagnosis, treatment strategies are determined based on symptom severity and duration.
Non-Surgical Management
Initial management of lumbar radiculopathy typically favors a conservative approach. This includes patient education about the condition, encouraging activity and exercise, manual therapy techniques such as McKenzie exercises, and pharmacological management, often starting with non-steroidal anti-inflammatory drugs (NSAIDs) as first-line agents [7, 8, 9]. McKenzie exercises have demonstrated efficacy in providing short-term symptomatic relief for some patients undergoing conservative treatment for lumbar radiculopathy [10]. Oral corticosteroids, administered as a tapering dose, may offer benefit in the acute phase of radiculopathy [11].
If initial conservative measures are insufficient, pain-relieving spinal injections are often considered. These may include epidural steroid injections, facet joint injections, or transforaminal epidural steroid injections, which have been shown to provide both short-term and, in some cases, long-term symptom relief [12]. These injections typically contain a combination of a glucocorticoid (an anti-inflammatory agent) and a long-acting local anesthetic like Marcaine.
Spinal injections can also serve a diagnostic purpose, particularly when the pain generator is uncertain. For example, in a patient presenting with predominant low back pain and minor foot numbness, extensive spinal arthritis, and who experiences significant pain relief from a facet joint injection, it suggests that facet joint arthropathy, rather than nerve root compression, is the primary pain source. Conversely, a patient with severe leg pain in an L5 dermatomal pattern who achieves substantial relief after an epidural injection is more likely to have nerve root compression as the pain generator.
Surgical Decision-Making
Surgical intervention becomes a consideration when conservative treatments fail to provide adequate symptom relief. The timeframe for considering conservative treatment as failed and proceeding to surgery is generally between four and eight weeks [13]. However, determining which patients will benefit most from surgery versus continued conservative care remains a subject of debate. The Spine Patient Outcomes Research Trial (SPORT) was a landmark study that addressed this question [14]. This trial randomized 501 patients with lumbar disc herniations to surgical or non-surgical treatment groups. The primary outcome measures were the SF-36 (a health survey) and the Oswestry Disability Index (ODI) scores assessed at various intervals. The SPORT trial found that both surgical and non-operative treatment groups showed substantial improvement over two years. While improvements were consistently numerically greater in the surgery group across all time points, the differences were often small and not statistically significant [14].
A key finding from the SPORT study was that most patients with lumbar radiculopathy improve over time, irrespective of whether they undergo surgery. However, at eight-year follow-up, subgroups of patients who derived the most benefit from surgical intervention included those with sequestered disc fragments, symptom duration exceeding six months, higher baseline low back pain levels, and those who were not working or disabled at baseline [4].
Ultimately, the decision regarding the timing of surgery is often individualized, based on the severity of the patient’s symptoms, clinical experience, and patient preferences. Overall, studies indicate that surgery tends to be more beneficial for patients with more severe radiculopathy symptoms [15].
Surgical Techniques for Radiculopathy
Discectomy remains the gold standard surgical procedure for lumbar disc herniation causing radiculopathy. The initial description of discectomy dates back to 1939 when Semmes described a subtotal laminectomy approach with dural sac retraction to remove the herniated disc [16]. Since then, numerous refinements have focused on developing less invasive techniques. Caspar and Williams reported on microsurgical discectomy techniques in 1977 and 1978 [17]. In 1997, Foley introduced microendoscopic discectomy (MED) [18, 19].
Current surgical options encompass open laminectomy with discectomy, “mini-open” hemilaminectomy with microdiscectomy, minimally invasive hemilaminectomy with microdiscectomy using tubular retractors, and MED. Studies comparing MED to open techniques have indicated that MED is associated with less nerve root irritation during surgery (as measured by intraoperative EMG) [20], reduced postoperative analgesic requirements, less blood loss, and shorter recovery times [21, 22]. Minimally invasive approaches may also lead to less joint destabilization and reduced surgical and hospital costs [22]. However, minimally invasive techniques have limitations, including a restricted surgical field of view and potential challenges in accessing pathology from different angles. The suitability of minimally invasive techniques is case-dependent and should be determined on an individual patient basis.
In traditional open discectomy, fluoroscopy is used for level localization, followed by a midline skin incision at the disc level. The incision is deepened to expose the lamina and ligamentum flavum. Retractors are placed, and a microscope is used for visualization. Hemilaminectomy and partial medial facetectomy are performed using a high-speed drill and Kerrison rongeurs. The ligamentum flavum is removed to expose the nerve root crossing the disc. The nerve root and thecal sac are retracted medially, and an incision is made in the annulus to remove disc material. A nerve hook can be used to explore for and remove any migrated disc fragments. Irrigation is used to flush out loose fragments. Open discectomy offers advantages such as enhanced visualization, a wider range of instrument use, and the ability to approach pathology from multiple trajectories, unlike the more constrained approaches of minimally invasive surgery (MIS).
For a MIS approach, a paramedian incision is made approximately 2 cm off the midline. A small incision is made in the lumbodorsal fascia, and a K-wire or tubular retractor is positioned on the facet joint at the disc level. Sequential dilators are used to create a muscle-splitting approach. A microscope or endoscope (for MED) is then used for visualization. Laminotomy, medial facetectomy, and microdiscectomy are performed similarly to the open technique.
Clinical Pearl:
In MIS discectomy, ensuring the tubular retractor trajectory is perpendicular to the target disc is critical. An incorrect trajectory can significantly hinder visualization and decompression of the nerve root. Intraoperative fluoroscopy is essential to confirm appropriate trajectory and positioning.
Conclusion
Lumbar radiculopathy remains a common neurological condition frequently evaluated by neurosurgeons. While the underlying pathology persists, advancements in spine surgery are continually leading to the development of less invasive surgical techniques. A thorough understanding of the clinical presentation, red flag symptoms, radiographic diagnosis, diagnostic tools, and both conservative and surgical treatment options is essential for clinicians managing this condition. Red flag symptoms that necessitate urgent evaluation include saddle anesthesia, bowel or bladder incontinence, and sudden extremity paresis.
Disclaimer: The content provided in this article is for informational and educational purposes only and does not constitute medical advice. Always consult with a qualified healthcare professional for diagnosis and treatment of medical conditions.
Footnotes
The authors have declared that no competing interests exist.
References
References
[1] de Schepper EI, Koes BW, van der Heijden GJ, et al. Sciatica with radiating pain: a systematic review within the framework of the International Classification of Functioning, Disability and Health. Eur Spine J. 2008;17(6):769-788. doi:10.1007/s00586-008-0633-x
[2] Tarasuk V, Goeree R, Burke TA, et al. The impact of sciatica: burden of illness, direct and indirect costs. Spine (Phila Pa 1976). 2005;30(18 Suppl):S29-S37. doi:10.1097/01.brs.0000179878.15899.1e
[3] Jensen RK, Kongsted A, Kjaer P, et al. Factors associated with persistent low back pain: a systematic review. Pain. 2011;152(1):1-17. doi:10.1016/j.pain.2010.07.032
[4] Dydyk AM, Khan MZ, Singh G. Straight Leg Raise Test. In: StatPearls. Treasure Island (FL): StatPearls Publishing; July 17, 2023.
[5] Preston DC, Shapiro BE. Electromyography and Neuromuscular Disorders: Clinical-Electrophysiologic Correlations. 3rd ed. Elsevier; 2013.
[6] Manchikanti L, Boswell MV, Singh V, et al. Comprehensive evidence-based guidelines for interventional techniques in the management of chronic spinal pain. Pain Physician. 2009;12(4):699-802.
[7] Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147(7):492-504. doi:10.7326/0003-4819-147-7-200710020-00008
[8] Koes BW, van Tulder MW, Ostelo RW, et al. Clinical guidelines for the management of sciatica. Scand J Work Environ Health. 2007;33(3):163-171.
[9] Roelofs PD, Deyo RA, Koes BW, et al. Cochrane review: Non-steroidal anti-inflammatory drugs for sciatica. Spine (Phila Pa 1976). 2008;33(17):1859-1867. doi:10.1097/BRS.0b013e3181809f8a
[10] Clare HA, Maher CG, O’Connell D, et al. Reliability of McKenzie classification of patients with chronic low back pain. Aust J Physiother. 2005;51(1):67-70.
[11] Weber KT, Werner BC, Bass JC, et al. The use of oral steroids in acute radiculopathy secondary to lumbar disc herniation: a systematic review and meta-analysis. Spine J. 2017;17(3):440-451. doi:10.1016/j.spinee.2016.09.017
[12] Arden NK, Price C, Reading I, et al. A multicentre randomized controlled trial of epidural corticosteroid injection for sciatica: the WEST study. Rheumatology (Oxford). 2005;44(11):1399-1406. doi:10.1093/rheumatology/kei124
[13] Atlas SJ, Keller RB, Robson D, et al. Surgical and nonsurgical management of sciatica secondary to lumbar disc herniation: five-year outcomes from the Maine Lumbar Spine Study. Spine (Phila Pa 1976). 2000;25(20):2596-2602.
[14] Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical vs nonoperative treatment for lumbar disc herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. JAMA. 2006;296(20):2441-2450. doi:10.1001/jama.296.20.2441
[15] Mirza SK, Mirza AJ, Chapman JR, et al. Lumbar discectomy versus nonoperative treatment for sciatica: a systematic review and meta-analysis. Eur Spine J. 2003;12(2):176-186. doi:10.1007/s00586-002-0479-x
[16] Semmes RE. Ruptured intervertebral discs: diagnosis and treatment, using a new surgical technique. South Med J. 1939;32(11):1027-1037.
[17] Caspar W. A new surgical procedure for lumbar disc herniation using an operating microscope. Acta Neurochir Suppl (Wien). 1977;24:52-53.
[18] Foley KT, Smith MM, Rampersaud YR. Microendoscopic discectomy. Tech Orthop. 1997;12(4):273-279.
[19] Perez-Cruet MJ, Foley KT, Isaacs RE, et al. Microendoscopic discectomy: technical note. Neurosurgery. 2002;51(Suppl 2):S129-S136.
[20] Perez-Cruet MJ, Patel NN, Foley KT. Endoscopic microdiscectomy: greater nerve root decompression with less muscle retraction. Surg Endosc. 2003;17(7):1107-1111. doi:10.1007/s00464-002-9190-9
[21] Arts MP, Brand R, van den Akker ME, et al. Tubular technique versus conventional microdiscectomy for sciatica: a randomized controlled trial. Neurosurgery. 2011;69(4):690-700; discussion 700-701. doi:10.1227/NEU.0b013e318224391d
[22] Overdevest GM, Jacobs WC, Vleggeert-Lankamp CL. Microendoscopic discectomy for lumbar disc herniation: a systematic review and meta-analysis of 14,838 patients. Eur Spine J. 2011;20(4):529-542. doi:10.1007/s00586-010-1634-7