Introduction
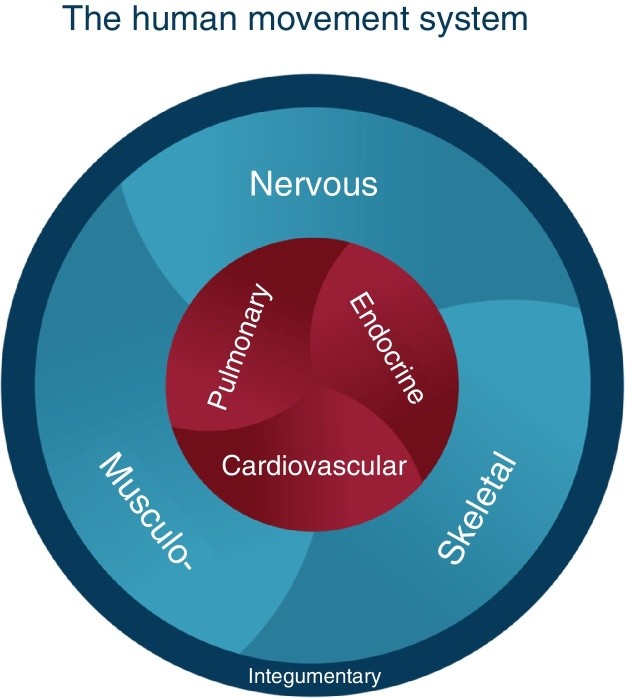
Since 1980, Shirley Sahrmann and her colleagues have pioneered the development of Movement System Impairment (MSI) syndromes, a diagnostic and treatment framework designed for physical therapists. This approach aims to guide treatment strategies and inform prognosis for a range of musculoskeletal conditions. The significance of the movement system in healthcare was underscored when the American Physical Therapy Association adopted it as the identity of physical therapy in 2013. Washington University defines the movement system as “a system of physiological organ systems that interact to produce movement of the body and its parts,” encompassing key components as illustrated in Fig. 1.
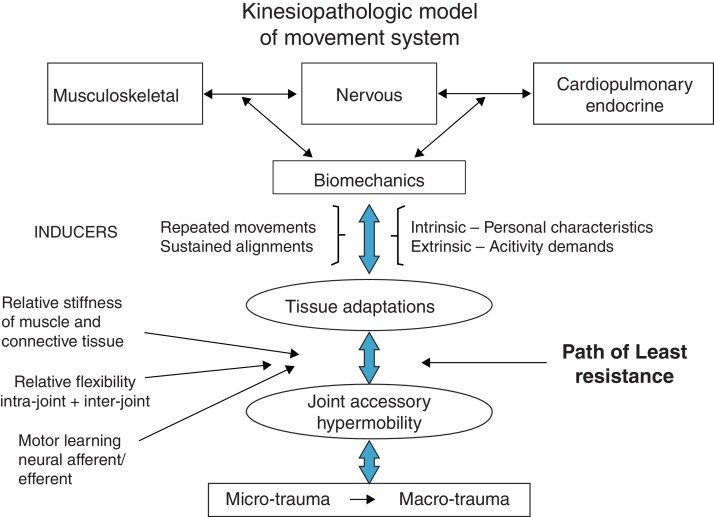
At the heart of MSI syndromes lies the kinesiopathologic model (KPM) (Fig. 2). This model operates on the fundamental principle that both repetitive movements and sustained postures can be primary contributors to musculoskeletal pathologies. MSI syndromes are theorized to arise from the cumulative effect of habitually using non-optimal alignments and movement patterns. Over time, these patterns become ingrained impairments, potentially leading to pathoanatomical changes within tissues and joint structures. The KPM highlights the interconnected roles of: (1) the musculoskeletal system as the effector of movement, (2) the nervous system as the regulator, and (3) the cardiovascular, pulmonary, and endocrine systems providing essential support and also being influenced by movement. For instance, the link between metabolic syndrome and insufficient physical activity is well-documented. The prevailing theory, supported by evidence, suggests that daily activities involving sustained alignments and repetitive movements are the main drivers of changes across these systems. These changes are further modulated by individual intrinsic factors, such as personal characteristics, and extrinsic factors, like the intensity and nature of physical activity at work and during leisure.
A crucial concept within MSI is that the body, particularly at the joint level, operates according to basic physics, favoring the path of least resistance in movement, often consistently in directions like flexion, extension, or rotation. This path is determined by: (1) relative flexibility both within and between joints, (2) the relative stiffness of muscles and connective tissues, and (3) the learned motor patterns that evolve into motor learning. When a joint consistently moves more easily in a specific direction, it can lead to the gradual development of hypermobility in accessory motions or micro-instability. This micro-instability can cause tissue microtrauma, which, with repeated stress, can escalate to macrotrauma.
Figure 1.
Alt Text: The human movement system graphic illustrating the interconnected physiological systems responsible for body movement, as developed by Washington University’s Physical Therapy Program.
Figure 2.
Alt Text: Kinesiopathologic model diagram showing the movement system impairment framework, highlighting the relationship between movement, alignment, and potential pathology.
The KPM framework suggests that detectable signs of movement impairments may precede the onset of noticeable symptoms. Furthermore, it posits that addressing and correcting these impaired alignments and movements, along with their contributing factors, represents the most effective strategy for managing musculoskeletal pain. This approach emphasizes identifying and treating the root cause of tissue injury, rather than solely focusing on the pathoanatomy of the affected tissues. Diagnosis within the MSI system begins by identifying specific impaired alignments and movements through a series of targeted clinical tests. These assessments often involve movements or positions that provoke or exacerbate the patient’s symptoms. The therapist then guides the patient in correcting these alignments and movements to assess whether symptom improvement occurs. Following a comprehensive examination, the information gathered is used to: (1) determine the specific MSI syndrome, (2) pinpoint contributing factors, (3) design corrective exercises, (4) identify necessary adjustments to alignments and movements during daily activities, and (5) educate the patient about the factors contributing to their condition, empowering them to practice corrections in their daily routines.
Consider the example of a patient diagnosed with Supraspinatus Tendinopathy. While tendinopathy indicates the pathoanatomical source of pain, an MSI assessment focuses on scapular and humeral alignment and movement patterns and their correlation with symptoms. A potential MSI diagnosis might be Scapular Insufficient Upward Rotation with Humeral Anterior Glide. Further examination would identify contributing factors such as (1) relative stiffness, (2) muscle strength imbalances, and (3) neuromuscular activation pattern deficits. The core principle of KPM is that classifying the patient based on these movement impairments (e.g., Scapular Insufficient Upward Rotation, Humeral Anterior Glide) provides more actionable guidance for physical therapy treatment than simply identifying a pathoanatomical diagnosis. Table 1 summarizes the key concepts underpinning the MSI syndromes.
Table 1.
Kinesiopathologic Model: Core Principles.
| Principle | Description |
|---|---|
| Cumulative Micro-trauma | Musculoskeletal pain syndromes often result from cumulative micro-trauma due to sustained postures or repetitive movements in specific directions during daily activities, leading to tissue stress and irritation. |
| Joint as Pain Generator | The joint that moves too readily in a particular direction becomes a primary site of pain generation due to instability and stress. |
| Path of Least Resistance | Micro-instability at a joint, combined with relative stiffness, neuromuscular activation patterns, and motor learning, contributes to establishing and maintaining a path of least resistance in movement, often exacerbating impairments. |
| Treatment Focus | Effective treatment aims to correct impaired alignments and movements that contribute to tissue irritation, as well as addressing secondary tissue adaptations like stiffness, weakness, and neuromuscular imbalances. |
| Integrated Training | Training that focuses on correcting impaired alignments and movements, rather than isolating individual muscles, promotes more effective neural and musculoskeletal adaptations and functional recovery. |
Relative Flexibility and Stiffness in MSI
Central to the MSI concept are the principles of relative flexibility and relative stiffness. Relative flexibility pertains to the joint itself. Intra-joint relative flexibility refers to hypermobility in accessory motions—spin, roll, or glide. This means one or more of these motions occurs too easily, resulting in an excessive range or frequency of motion. Inter-joint relative flexibility describes a scenario where motion occurs more readily in one joint of a joint pair, even when it should ideally occur in the adjacent joint. A classic example is during forward bending, where the lumbar spine may flex excessively compared to the hips.
Stiffness, conversely, is defined as the resistance encountered during passive elongation of muscle and connective tissue. Stiffness is influenced by muscle hypertrophy and collagen content. Viscosity also plays a role, particularly dependent on the speed of movement. Movement, adhering to physical laws, will always seek the path of least resistance. The determinants of this path are (1) relative flexibility, (2) relative stiffness, and (3) motor learning. When movement involves multiple joints, the body naturally favors increasing motion at the joint with less resistance or lower stiffness compared to a joint with greater resistance or stiffness. For instance, during hip extension, the lumbar spine might extend more readily than the hip joint itself. Relative flexibility impairments can also manifest in single-joint movements. For example, during seated knee extension, early posterior pelvic tilt and lumbar flexion suggest a relative flexibility impairment in the lumbar spine, indicating stiffer hamstring muscles compared to back extensors.
Inducers and Modifiers of Movement System Impairments
Sustained postures and repetitive movements inherent in daily activities are considered the primary inducers of tissue adaptations and the impaired alignments and movements characteristic of MSI syndromes. For instance, individuals engaged in activities with high rotational demands often exhibit increased lumbopelvic rotation compared to those who are not. Numerous studies have demonstrated that repetitive movements associated with various sports and occupations lead to tissue adaptations across bones, joints, and surrounding tissues, including muscles.
However, the impact of sustained alignments and repetitive movements on tissue adaptation and symptom development is modified by a variety of factors. These include age, gender, tissue mobility, anthropometrics, activity level, and psychological factors. Older individuals may exhibit different responses to repetitive movements compared to younger people due to age-related joint and tissue degeneration. Pain sensitivity also varies with age. Gender differences in alignment may also influence the effects of movement patterns. For example, men and women with low back pain may present with different pain-provoking alignments and movements. Women tend to exhibit increased knee abduction during weight-bearing activities, which can elevate their risk of patellofemoral pain and ACL injuries. Tissue mobility itself is a modifier; joint hypermobility can reduce proprioception and increase the risk of musculoskeletal conditions. Anthropometric factors, such as femoral neck shaft angle in women, can predispose individuals to conditions like greater trochanteric pain syndrome. Body structure, like a long trunk, may contribute to depressed shoulder alignment and reduced pain threshold in the upper trapezius. Activity levels also play a modifying role; both inadequate and excessive physical activity can increase musculoskeletal risk, while appropriate activity levels are protective. Finally, psychological factors can significantly modulate pain intensity and outcomes in musculoskeletal conditions like tendinopathy, low back pain, and post-ACL reconstruction.
Alignment and Movement Impairments: Symptomatic vs. Asymptomatic Individuals
The core principle of KPM is to restore optimal alignment and correct movement impairments. While some studies have not found significant differences in alignment and movement patterns between healthy individuals and those with musculoskeletal symptoms, numerous others have identified notable distinctions. For example, patellofemoral pain is associated with increased peak hip adduction, internal rotation, and contralateral pelvic drop. Kinematic studies of the shoulder complex have revealed clear differences between individuals with and without shoulder pain. Sitting posture has been linked to upper quadrant musculoskeletal pain experienced during sitting. Individuals with femoroacetabular impingement exhibit altered pelvic movement patterns during hip flexion compared to healthy controls. People with low back pain tend to move their lumbopelvic region more extensively and earlier during lower limb movements than those without back pain.
Importantly, research also indicates that certain alignment and movement impairments observed in asymptomatic individuals may actually increase their future risk of developing musculoskeletal pain. For example, lumbopelvic movement impairments during hip abduction and standing with excessive lumbar lordosis have been identified as potential risk factors for low back pain development, especially in professions requiring prolonged standing.
MSI Examination and Classification Process
The MSI examination and classification process involves a detailed interpretation of data collected from a series of alignment and movement tests. During these tests, clinicians assess the timing and magnitude of movements, the degree of end-range alignment in specific joints, and the impact on the patient’s symptoms. Symptom-provoking tests are immediately followed by systematic corrections of the identified impairment to determine its direct role in the patient’s symptoms. Correction strategies typically involve: (1) minimizing excessive or premature movement, especially accessory motions, in the symptomatic joint while promoting movement in adjacent joints, or (2) reducing end-range alignment positions in specific directions. A positive response, indicated by symptom improvement upon correction, confirms the association of the identified alignment or movement impairment with the patient’s symptoms.
MSI syndromes have been comprehensively developed for all major body regions, including the cervical, thoracic, and lumbar spine, shoulder, elbow and hand, hip, knee, and ankle and foot (Table 2).
Table 2.
MSI Syndromes Categorized by Body Region.
| Body Region | Syndrome Examples |
|---|---|
| Cervical Spine | Cervical Extension, Cervical Extension-Rotation, Cervical Flexion, Cervical Flexion-Rotation, Cervical Rotation |
| Thoracic Spine | Thoracic Rotation-Flexion, Thoracic Flexion, Thoracic Rotation-Extension, Thoracic Rotation, Thoracic Extension |
| Shoulder | Scapular Insufficient Upward Rotation, Scapular Internal Rotation, Scapular Depression, Scapular Abduction, Scapular Adduction, Scapular Winging and Tilting, Humeral Anterior Glide, Humeral Superior Glide, Shoulder Medial Rotation Glenohumeral Hypomobility |
| Elbow | Wrist Extension with Forearm Pronation, Elbow Hypomobility, Elbow Flexion, Elbow Valgus, Elbow Extension, Wrist Flexion with Forearm Pronation Elbow Impairment |
| Wrist and Hand | Insufficient Finger and/or Thumb Flexion, Insufficient Finger and/or Thumb Extension, Insufficient Thumb Palmar Abduction and/or Opposition, Thumb Carpometacarpal Accessory Hypermobility, Finger or Thumb Flexion with or without Finger Rotation, Source or Regional Impairment of the Hand |
| Lumbar Spine | Lumbar Flexion, Lumbar Extension, Lumbar Rotation, Lumbar Rotation with Flexion, Lumbar Rotation with Extension |
| Hip | Femoral Anterior Glide, Femoral Anterior Glide with Medial Rotation, Femoral Anterior Glide with Lateral Rotation, Femoral Posterior Glide, Femoral Multidirectional Accessory Hypermobility, Femoral Hypomobility with Superior Glide, Hip Adduction, Hip Adduction with Medial Rotation, Femoral Lateral Glide, Hip Extension with Knee Extension, Hip Extension with Medial Rotation, Hip Lateral Rotation |
| Knee | Tibiofemoral Rotation, Tibiofemoral Hypomobility, Knee Extension, Knee Extension with Patellar Superior Glide, Knee Hyperextension, Patellar Lateral Glide Knee Impairment |
| Foot and Ankle | Pronation, Supination, Insufficient Dorsiflexion, Hypomobility, Foot and Ankle Impairment, Proximal Tibiofibular Glide |
Validity and Reliability of MSI Syndromes
The validity of MSI syndromes has been investigated in several studies, primarily focusing on the lumbar and knee regions. Research has reported partial construct validity for MSI syndromes proposed for these areas. Other studies have compared movement impairments and associated signs and symptoms across different MSI syndromes. For example, research has shown that individuals with Lumbar Rotation with Extension Syndrome exhibit distinct asymmetric lumbar movement patterns during trunk lateral flexion compared to those with Lumbar Rotation Syndrome. Systematic differences in hip and lumbopelvic movement during active hip lateral rotation tests have also been observed between individuals with Lumbar Rotation Syndrome and Lumbar Rotation with Extension Syndrome. Furthermore, individuals with Lumbar Rotation with Flexion Syndrome have been found to demonstrate greater lumbar flexion during trunk flexion tests compared to those with Lumbar Rotation with Extension Syndrome. In slumped sitting, individuals with Lumbar Rotation Syndrome showed greater end-range lumbar flexion compared to those with Lumbar Rotation with Extension Syndrome.
The reliability of clinicians in classifying patients into MSI syndromes has also been evaluated, particularly for the lumbar spine and knee. Studies indicate that clinicians can reliably classify patients into lumbar MSI syndromes, even with varying levels of clinical experience. Reliability assessments for knee MSI syndromes have shown moderate to substantial intra-rater and inter-rater reliability for classification judgments.
Treatment Strategies Based on MSI Syndromes
Treatment within the MSI framework encompasses patient education, detailed analysis and modification of daily activities, and the prescription of specific corrective exercises. Patient education is crucial for helping individuals understand how repetitive impaired movements and sustained non-optimal postures can contribute to their musculoskeletal conditions. Patients are taught how to recognize and correct these impairments in their daily routines, especially during activities that trigger symptoms. For instance, someone with Scapular Depression Syndrome might be instructed to maintain scapular elevation by using arm supports while working at a computer, thereby reducing sustained load on the cervical spine and scapular elevator muscles.
A cornerstone of MSI treatment is teaching patients to perform their daily activities correctly and without provoking symptoms. Since sustained alignments and repetitive movements are considered causal factors, their correction is paramount. This correction process also enhances patient awareness of symptom triggers and empowers them to manage or minimize these symptoms. Patients are encouraged to apply these corrections throughout their day. Recent research highlights that, for individuals with low back pain, greater adherence to corrected daily activities, compared to exercise adherence, is linked to more significant improvements in function, pain levels, and other relevant outcomes.
Exercise prescription in MSI is syndrome-specific and targets the contributing factors identified during the initial examination. Exercises focus on practicing the correction of impaired alignments and movements that were pinpointed during clinical tests. For example, a patient with Hip Adduction Syndrome who demonstrates excessive hip adduction and associated hip pain during a partial squat test would use the squat movement itself as a corrective exercise. The focus would be on modifying the amount and timing of hip adduction during the squat to promote correct movement patterns.
These specific exercises and activity modifications are practiced both during therapy sessions and as part of a home exercise program. Patients are typically provided with visual aids, such as pictures or diagrams of exercises and corrected daily activities, along with written instructions. Videos can also be used to enhance understanding and proper execution. The patient’s ability to perform their program correctly is continually assessed during follow-up visits to guide program progression. Evaluating the patient’s grasp of the core principles behind each exercise or activity and their independence in performing them is vital for making informed decisions about when and how to progress the treatment plan.
Clinical Evidence for MSI Treatment
Numerous case reports have documented the application of MSI treatment across a range of conditions, including shoulder pain, low back pain, abdominal pain, cervicogenic headache, and knee pain. A feasibility randomized clinical trial has also explored MSI treatment for chronic hip pain. A randomized controlled trial specifically investigating the treatment of chronic low back pain compared the effectiveness of a Classification-Specific (CS) treatment approach (based on MSI principles) to a non-Classification-Specific (NCS) treatment. Both CS and NCS treatments included exercise and daily activity modification. The CS treatment incorporated education, exercise, and daily activity correction aligned with MSI syndromes. The NCS treatment focused on education and daily activity correction emphasizing the maintenance of a neutral spine, with exercises aimed at trunk strengthening and flexibility in the trunk and lower limbs. Interestingly, the study found no significant difference in efficacy between the CS and NCS approaches. The authors suggested that the comparable improvements observed in both groups might be attributed to the shared emphasis on daily activity correction, particularly the focus on maintaining a neutral spine and promoting movement in other joints during daily tasks. This interpretation was further supported by the finding that participants in both groups demonstrated greater adherence to daily activity corrections than to prescribed exercises.
Conclusion
The MSI-based classification and treatment system provides physical therapists with a robust framework for diagnosing and treating musculoskeletal conditions rooted in the principles of the Kinesiopathologic Model. This model posits that impaired alignments and movements are primary contributors to pain and pathology. MSI syndromes and corresponding treatment protocols have been established for all major body regions. The reliability and validity of the MSI system have been partially substantiated for certain anatomical areas. Numerous case reports detail the MSI examination and treatment of diverse musculoskeletal conditions. While case evidence is supportive, further randomized controlled trials are needed to rigorously evaluate the efficacy of MSI syndrome treatment, particularly beyond chronic low back pain where some RCT evidence exists. Additional research in this area will be crucial to fully establish the evidence-based effectiveness of Shirley Sahrmann’s Movement System Impairment approach in physical therapy.
Conflicts of interest
Daniel Azevedo is an instructor in continuing education courses that include MSI content.
Shirley Sahrmann teaches continuing education courses on MSI and receives royalties from her book “Diagnosis and Treatment of Impairment Syndromes” published by Elsevier.
Linda Van Dillen declares no conflicts of interest.
Acknowledgements
This work was partially supported by NIH/NICHD/NCMRR Grant number HD 047709 (PI: Van Dillen).
References
1 Sahrmann SA. Diagnosis and treatment of movement impairment syndromes. St. Louis: Mosby; 2002.
2 Sahrmann SA. Movement system impairment syndromes of the extremities, cervical and thoracic spines. St. Louis: Mosby; 2011.
3 Ford ES, Kohl HW 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and metabolic syndrome in obese and nonobese adults. Arterioscler Thromb Vasc Biol. 2005;25(8):1751-7. DOI
4 Carter DR, Beaupré GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration, remodeling, and maintenance. In: Hall JE, editor. Guyton and Hall textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders; 2006. p. 855-72.
5 Frost HM. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081-101. DOI
6 Kapandji IA. The physiology of the joints. Vol 2. 5th ed. London: Churchill Livingstone; 1996.
7 Woo SL, Hildebrand KA, Frank CB, Debnath J, Guo XE, Bray RC. Mechanobiology of joint structures. In: Hall JE, editor. Guyton and Hall textbook of medical physiology. 11th ed. Philadelphia: Elsevier Saunders; 2006. p. 873-92.
8 Kim K, Lee AS, Lee B, Yoon JO, Kim Y, Lee HY, et al. Differences in lumbar and hip motion during trunk flexion in subgroups of movement impairment syndrome for the lumbar spine. Man Ther. 2017;27:133-9. DOI
9 Purslow PP. The structure and function of muscle connective tissue. In: Garamszegi N, editor. Muscle and tendon injuries. London: Springer; 2014. p. 27-52. DOI
10 Franchi MV, Reeves ND, Narici MV. Skeletal muscle remodeling in response to eccentric vs. concentric loading: morphological, molecular, and metabolic adaptations. J Appl Physiol (1985). 2017;123(6):1817-27. DOI
11 Gillies JH, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44(3):318-31. DOI
12 Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Philos Trans R Soc Lond B Biol Sci. 2011;366(1570):1466-76. DOI
13 Maas C, Sandner B, Chaudhry H, Klingler W. Myofascial force transmission to the upper limb is reduced after experimental induction of latent myofascial trigger points in the rat latissimus dorsi muscle. J Bodyw Mov Ther. 2015;19(1):130-6. DOI
14 Chaudhry H, Schleip R, Ji Z, Bukiet B, Maney M, Findley TW. Three-dimensional mathematical model for deformation of human fasciae in manual therapy. J Am Osteopath Assoc. 2008;108(8):379-90.
15 Chaudhry H, Findley TW, Quigley KS, Ji Z, Sims-Williams H, Daepp R, et al. Measures of tissue stiffness and elastic energy storage in lumbar connective tissue. J Manipulative Physiol Ther. 2012;35(3):171-8. DOI
16 Nadler SF, Malanga GA, Bartoli LA, Feinberg JH, Prybicien M, DePrince M. Hip muscle imbalance and low back pain in athletes: relationship of hip and core muscle strength to pain in athletes with chronic low back pain. Am J Sports Med. 2002;30(5):749-54. DOI
17 Zdziarski LA, Van Dillen LR, Stevens MJ, Lewek MD, Gill NW. Movement impairments in people with chronic low back pain: systematic review and meta-analysis. J Orthop Sports Phys Ther. 2017;47(11):802-48. DOI
18 Dieppe PA, Lohmander LS. Osteoarthritis. Lancet. 2005;365(9463):1502-11. DOI
19 Szeto GP, Lee R, Sit JW, Cheung PW, Tiu WK, Wong WT, et al. Head and shoulder posture affect upper trapezius myofascial trigger point sensitivity in office workers. Man Ther. 2016;25:e1-9. DOI
20 Gillam I, Gibberd M, Baber M, Grahame R, Bird HA. The effect of joint hypermobility on muscle strength. Br J Rheumatol. 1995;34(10):950-3. DOI
21 Herrero P, Sindrup SH, Mostgaard G, Andersen J, Petersen P, Jensen TS. Pain and somatosensory function in chronic painful polyneuropathy and healthy subjects. Pain. 1998;74(2-3):205-18. DOI
22 Johnston WT, Howard JS, Kaufman KR. Gender differences in lower extremity kinematics during walking. Gait Posture. 2012;36(3):437-42. DOI
23 Keays KS, Jabbour L, Friedman S, Backman C, Riseborough S, Visentini PJ, et al. Psychological factors predict pain and disability after anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(1):99-105. DOI
24 Lequesne M, Mathieu P, Poquet M, Revel M, Maigne JY. [Greater trochanteric pain syndrome. An evaluation of ischiotrochanteric bursography]. Rev Rhum Mal Osteoartic. 1994;61(11):747-54.
25 Lautenbacher S, Kunz M, Weber N, Stuber F, Zimmer HG, Sprenger T, et al. Age differences in heat pain, cold pain, and heat pain sensitization. Pain. 2005;115(1-2):147-56. DOI
26 Nakagawa TH, Moriya ET, Maciel CD, Serrão FV. Trunk, hip, and lower limb muscle strength in women with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2008;38(10):657-66. DOI
27 Reuter SE, Davis IS. Altered movement patterns in runners with chronic hip pain. J Biomech. 2012;45(5):939-44. DOI
28 Roussouly P, Gollogly S, Berard R, Noseda R, Fehlings MG, Deprest S. Sagittal alignment of the spine and pelvis during growth. Eur Spine J. 2013;22(4):809-18. DOI
29 Sadiya T, Bader DL. Excessive mechanical loading of the musculoskeletal system in bed-ridden patients: implications for pressure ulcer development. J Tissue Viability. 2017;26(4):226-33. DOI
30 Schiphorst Preuper HR, Reneman MF, Dijkstra PU. Psychological factors and tendinopathy: a systematic review. J Pain Res. 2017;10:491-502. DOI
31 Smith BE, Hendrick P, Smith TO, Bateman M, Moffatt F, Rathleff MS, et al. Should exercises be painful in the management of chronic musculoskeletal pain? A systematic review and meta-analysis. Br J Sports Med. 2017;51(23):1679-87. DOI
32 Teyhen DS, Flynn TW, Childs JD, Abraham LD, Wright AC, Davee AM, et al. Differences in movement impairments between female and male subjects with chronic low back pain. Clin J Pain. 2007;23(8):731-8. DOI
33 Van Rijn RM, Van Os JG, Kleijnen J, Verbunt JA, Hazes JM, Janssens L. Hypermobility associated with musculoskeletal complaints: a systematic review of the literature. J Orthop Sports Phys Ther. 2006;36(3):189-204. DOI
34 Vlaeyen JW, Linton SJ. Fear-avoidance model of chronic pain: 20 years on. Pain. 2012;153(6):1144-7. DOI
35 Buckwalter JA, Lane Smith R, Coates JE, Moore KC, Schumacher BL, Diduch DR, et al. Articular cartilage: composition, structure, and function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic basic science: foundations of clinical practice. Rosemont: American Academy of Orthopaedic Surgeons; 2000. p. 465-84.
36 Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: role of fixed charges and water. In: Mow VC, Hayes WC, editors. Basic orthopaedic biomechanics. 2nd ed. Philadelphia: Lippincott-Raven; 1997. p. 207-56.
37 Shultz SJ, Schmitz RJ, Nguyen AD, Chaudhari AM, Padua DA. Differences in hip and knee kinematics and kinetics between sexes during a drop jump. J Athl Train. 2015;50(2):176-83. DOI
38 van Heuvelen MJ, Frings-Dresen MH, Blatter BM, Bongers PM, van der Beek AJ. Dose-response relationship between physical activity and low back pain: an updated systematic review of prospective studies. Occup Environ Med. 2010;67(9):601-9. DOI
39 Dankaerts W, O’Sullivan P, Burnett A, Straker L, Bleakley C, Dane K, et al. Discriminating healthy controls and chronic low back pain patients in prone using trunk muscle activity during the active straight leg raise test. Man Ther. 2006;11(2):133-41. DOI
40 Park G, Lee SM. Comparison of muscle activity and spinal posture during sitting and standing in subjects with and without chronic low back pain. J Back Musculoskelet Rehabil. 2015;28(4):737-44. DOI
41 Casartelli NC, Leunig M, Maffiuletti NA. Hip muscle weakness in patients with symptomatic femoroacetabular impingement. Osteoarthritis Cartilage. 2011;19(7):816-22. DOI
42 Claus AP, Hides JA, Moseley GL, Hodges PW. Sitting posture and paraspinal muscle activity in people with chronic low back pain. Man Ther. 2009;14(2):194-200. DOI
43 Lamontagne M, Kennedy MJ, Beaulé PE. The effect of cam femoroacetabular impingement on hip and pelvic motion during gait. Gait Posture. 2011;33(4):659-63. DOI
44 Ludewig PM, Cook TM. Alterations in shoulder kinematics and associated muscle activity in people with symptoms of shoulder impingement. Phys Ther. 2000;80(3):276-91.
45 McClure PW, Michener LA, Sennett BJ, Karduna AR. Direct 3-dimensional assessment of scapular kinematics during dynamic movements in vivo. J Shoulder Elbow Surg. 2001;10(3):269-77. DOI
46 Souza RB, Powers CM. Predictors of hip internal rotation during running: an evaluation of hip muscle strength and flexibility. Am J Sports Med. 2009;37(8):1525-30. DOI
47 Willigenburg NW, Kingma I, Delecluse C, de Vries J, Pool-Goudzwaard AL, Meskers CG, et al. Differences in multi-segmental trunk coordination between patients with chronic low back pain and healthy controls during a lifting task. Clin Biomech (Bristol, Avon). 2014;29(1):94-101. DOI
48 Yoshimoto H, Cooper C, успехов JM, проверенных WC, успехов СМ, успехов WC. Range of motion of the hip and pelvis during gait in patients with acetabular dysplasia. J Orthop Res. 2012;30(1):79-83. DOI
49 Bullock-Saxton JE, Janda V, Bullock MI. Reflex lumbar erector spinae activity during the hip abduction test of Janda. Man Ther. 1993;1(1):3-10. DOI
50 McGill SM, Grenier S, Kavcic N, Cholewicki J. Coordination of muscle activity to assure stability of the lumbar spine. J Electromyogr Kinesiol. 2003;13(4):353-9. DOI
51 Van Dillen LR, Sahrmann SA, Norton BJ, McDonnell MK, Bloom NJ, Fleming DA, et al. Movement system impairment-based categories for low back pain: stage 1 validation. J Man Manip Ther. 2003;11(4):224-33. DOI
52 Van Dillen LR, Whitman JM, Boyles RE, Zabcik S, Gilliam J, McDonnell MK, et al. Movement system impairment-based categories for low back pain: stage 2 validation. J Man Manip Ther. 2007;15(3):164-73. DOI
53 Sahrmann SA. Movement system impairment syndromes: rationale and methods of treatment of movement impairment syndromes. In: Twomey LT, editor. Physical therapy of the spine and extremity. 3rd ed. London: Churchill Livingstone; 1999. p. 169-92.
54 Comerford MJ. Stabilisation strategies in painful and pain-free subjects. In: Jull G, Janda V, Comerford MJ, editors. Managing neck pain and headaches. London: Butterworth-Heinemann; 2000. p. 193-207.
55 Janda V. Muscle weakness and inhibition (in relation to pain). In: Boyling JD, Palastanga N, editors. Grieve’s modern manual therapy. 2nd ed. London: Churchill Livingstone; 1994. p. 361-8.
56 Gombatto SP, Hayes D, Junker D, Farrokhi S, Cook CE, Whitman JM. Differences in movement pattern and pain response in subgroups of movement impairment syndrome for the lumbar spine. J Orthop Sports Phys Ther. 2012;42(2):120-30. DOI
57 Gombatto SP, Sahrmann SA, Abbaszadeh S, Powers CM. Movement system impairment subgroups for low back pain: prevalence and discriminant validity. J Man Manip Ther. 2007;15(3):174-83. DOI
58 Herrington L. Differences in lumbar lordosis and pelvic tilt within subgroups of low back pain. J Man Manip Ther. 2011;19(4):221-7. DOI
59 Hides JA, Richardson CA, Jull GA. Multifidus muscle recovery is not automatic after resolution of acute, first-episode low back pain. Spine (Phila Pa 1976). 1996;21(23):2763-9. DOI
60 Ibrahim M, Giebert J, Dunn A, Liebenson C, Schneider M. Interrater reliability of the movement system impairment-based classification system for the lumbar spine. J Man Manip Ther. 2017;25(1):42-9. DOI
61 Karayannis NV, Jull GA, Hodges PW. Movement patterns of the lumbar spine during sit-to-stand and stand-to-sit in people with chronic low back pain. Spine (Phila Pa 1976). 2008;33(24):E907-14. DOI
62 Kelley LA, Shrout PE, Likert M, Seiber SE, Romanowski C, Domholdt E. Reliability of movement pattern classification in people with chronic low back pain. J Orthop Sports Phys Ther. 2013;43(1):4-12. DOI
63 Kulig K, Powers CM, Landel R, Chen CY, Fredericson M, Powers CM. Segmental lumbar motion during a modified curl-up in subjects with and without chronic low back pain. Phys Ther. 2004;84(10):889-901.
64 Lee AS, Kang TW, Kim K, Kim Y, Yoon JO, Lee HY, et al. Differences in spinal posture and trunk muscle activity during slumped sitting in subgroups of movement impairment syndrome for the lumbar spine. Man Ther. 2017;28:96-102. DOI
65 Pool JJ, Ostelo RW, de Vet HC, Brosschot JF, Vlaeyen JW, Struyf PC, et al. The relationship between static lumbar lordosis and pain: a systematic review of the literature. Spine (Phila Pa 1976). 2007;32(17):E480-91. DOI
66 Shirey ME, исследование H, исследование H, исследование H, исследование H, исследование H, et al. Lumbo-pelvic movement patterns during active hip abduction in people with low back pain compared to healthy controls. Eur Spine J. 2016;25(4):1222-9. DOI
67 Teyhen DS, Gill NW, Whittaker JL, Henry SM, Hides JA, Hodges PW. Paraspinal muscle size and composition in military personnel with chronic low back pain. Spine (Phila Pa 1976). 2011;36(13):E847-54. DOI
68 Van Dillen LR, McDonnell MK, Lowe CJ, Bayard C, Gilliam J, Whitman JM. Movement and alignment characteristics of the classification-based system for people with low back pain. Man Ther. 2008;13(1):32-43. DOI
69 Van Dillen LR, Whitman JM, Schneider MJ, Strube MJ, Flynn TW, Henderson CN, et al. Responsiveness of pain intensity and physical function measures in people with chronic low back pain classified using movement impairment-based categories. Pain. 2013;154(2):255-61. DOI
70 Werneke MW, Hart DL. Categorizing patients with occupational low back pain by use of the movement system impairment-based classification approach. Phys Ther. 2003;83(2):185-98.
71 Kaikkonen P, Airaksinen O, Hänninen O. Movement and muscle imbalance of the knee and hip in patellofemoral pain syndrome. Physiother Theory Pract. 2003;19(3):161-78. DOI
72 Ford JJ, Story J, Kelly BT, Sherman J, Gill NW. Inter-rater reliability of a movement system impairment-based classification system for the lumbar spine in novice raters. Man Ther. 2017;30:171-6. DOI
73 Ford JJ, Van Dillen LR, Flynn TW, Wainner RS, Gill NW. Interrater reliability of selected clinical tests for classification using a movement impairment-based diagnostic system for low back pain. Phys Ther. 2011;91(5):744-58. DOI
74 Rabin A, Irrgang JJ, Fitzgerald GK, Childs JD. Interrater reliability of selected clinical tests and measures and a movement impairment-based classification system used to examine people with chronic low back pain. Phys Ther. 2011;91(6):917-29. DOI
75 Van Dillen LR, Bloom N, Norton BJ, McDonnell MK, Cibulka MT, редактор МТ. Reliability of classifying patients with low back pain using a movement impairment-based classification system. Phys Ther. 2001;81(7):1252-62.
76 Werneke MW, Hart DL, George SZ, Fritz JM. Reliability of classifying patients with activity limitation and pain using the ICF framework. Phys Ther. 2005;85(5):447-59.
77 Whitman JM, Flynn TW, Childs JD, Wainner RS, Gill NW, редактор NW. The use of the ICF framework to classify a sample of patients with chronic low back pain. Phys Ther. 2004;84(2):143-65.
78 Kaibafvala CB, Ford JJ, Hahne AJ, Gill NW. Reliability of a movement system impairment-based classification system for the knee. Man Ther. 2019;44:213-8. DOI
79 Allison SC, двигатель SC, двигатель SC, двигатель SC. Management of movement system impairment syndromes: case studies. Aust J Physiother. 2000;46(3):223-30. DOI
80 Allison SC, двигатель SC, двигатель SC, двигатель SC. The diagnosis-specific approach versus the movement impairment approach to the management of patients with chronic low back pain. Man Ther. 2002;7(3):147-54. DOI
81 Allison SC, двигатель SC, двигатель SC, двигатель SC. The influence of direction of movement and velocity on the reported intensity and unpleasantness of chronic low back pain. Man Ther. 2003;8(3):153-61. DOI
82 Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA. Muscles testing and function with posture and pain. 5th ed. Baltimore: Lippincott Williams & Wilkins; 2005.
83 Neumann DA. Kinesiology of the musculoskeletal system: foundations for physical rehabilitation. 2nd ed. St. Louis: Mosby Elsevier; 2010.
84 Van Dillen LR, McDonnell MK, Holden MA, Peterson D, Coronado RA, Schneider MJ, et al. The effectiveness of classification-based treatment compared to non-classification-based treatment for chronic low back pain: a systematic review and meta-analysis. J Orthop Sports Phys Ther. 2020;50(5):225-41. DOI
85 Mathieson G. An approach to movement impairment syndromes: development of a clinical reasoning and treatment model. Aust J Physiother. 1999;45(4):277-83. DOI
86 Lasinski M, Jette DU, конструировать DU. Responsiveness of the patient-specific functional scale in patients with shoulder pain. J Orthop Sports Phys Ther. 2001;31(6):291-303. DOI
87 Stratford PW, конструировать PW, конструировать PW, конструировать PW. Assessing disability and change on individual patients: a report of a patient with knee dysfunction. Physiother Can. 1994;46(4):265-9. DOI
88 Teyhen DS, McClure PW, конструировать PW, конструировать PW. The use of surface electromyography to assess the validity of a movement impairment-based classification system. Man Ther. 2005;10(2):147-57. DOI
89 Torelli P, конструировать P, конструировать P, конструировать P. Cervicogenic headache and active physical therapy: a case report. J Man Manip Ther. 2003;11(2):115-24. DOI
90 Urschel S, конструировать S, конструировать S, конструировать S. Abdominal muscle training and movement impairment syndromes. J Bodyw Mov Ther. 2008;12(2):107-14. DOI
91 Lin CY, конструировать CY, конструировать CY, конструировать CY. Feasibility of a movement system impairment-based classification system for patients with chronic hip pain: a pilot study. J Man Manip Ther. 2017;25(4):235-43. DOI
92 Van Dillen LR, McDonnell MK, конструировать MK, конструировать MK. A classification-based approach to treatment of chronic low back pain: subgrouping patients with direction preference. Man Ther. 2003;8(4):252-62. DOI