Introduction
Testosterone Deficiency Syndrome (TDS), also known as hypogonadism or late-onset hypogonadism (LOH), is a condition characterized by insufficient levels of serum testosterone in men. This deficiency can occur with or without a decrease in androgen receptor sensitivity. TDS is not merely a condition of aging; it’s a significant health concern linked to various comorbidities, including cardiovascular disease, osteoporosis, diabetes, sexual dysfunction, sarcopenia, emotional disturbances, and metabolic syndrome. Despite established guidelines for diagnosis and management, a surprisingly small percentage of men with TDS receive appropriate care, highlighting a critical gap in healthcare. This article aims to provide an in-depth guide to Tds Diagnosis, drawing on expert consensus and current best practices to empower healthcare professionals and individuals seeking information.
Reduced testosterone levels can be triggered by various factors, including certain medications (like ketoconazole, spironolactone, estrogens, and methadone) and underlying health conditions such as diabetes, hypothyroidism, chronic obstructive pulmonary disease (COPD), obesity, hemochromatosis, and metabolic syndrome (MetS). Furthermore, testosterone levels naturally decline with age, making older men more susceptible to clinically relevant TDS. With life expectancy increasing in North America, the prevalence of TDS is expected to rise, further emphasizing the need for improved diagnostic and management strategies. Current estimates suggest that a significant proportion of men over 40, approximately 25% of those aged 40 to 62 in Canada, are biochemically testosterone deficient.
While recent guidelines and recommendations for TDS diagnosis and management are available, their underutilization is evident, with less than 10% of affected men receiving testosterone therapy. Several barriers contribute to this gap, including limited physician awareness of TDS associations with conditions like MetS, diabetes, and cardiovascular disease, and the potential benefits of testosterone replacement therapy (TRT) in mitigating these conditions. Unfounded concerns regarding prostate health and insufficient dissemination of existing guidelines also play a role. To address these challenges, a multidisciplinary panel of experts convened to enhance TDS knowledge among healthcare providers. This article synthesizes their key recommendations into a practical guide, focusing on detection, diagnosis, and management algorithms for TDS.
Detection and Screening for TDS: Identifying Candidates for TDS Diagnosis
Effective TDS diagnosis starts with targeted screening of men at higher risk. Certain clinical conditions are strongly associated with low testosterone levels, making men with these conditions prime candidates for screening. The correlation between diabetes and TDS is particularly notable, with approximately one-third of men with diabetes experiencing hypogonadism. Conversely, higher testosterone levels have been linked to a reduced risk of developing type 2 diabetes. Reflecting this connection, guidelines from organizations like the Canadian Diabetes Association and the Endocrine Society recommend screening for testosterone deficiency in all men with type 2 diabetes, and specifically for erectile dysfunction (ED), a common symptom of TDS, in diabetic men.
Clinical disorders associated with a high prevalence of low T levels:
| Type II diabetes mellitus |
|---|
| Metabolic syndrome |
| Human immunodeficiency virus-associated weight loss |
| Treatment with opioids, glucocorticoids or ketoconazole |
| Osteoporosis or low trauma fracture at a young age |
| End-stage renal disease and maintenance hemodialysis |
| Chronic obstructive pulmonary disease |
| Infertility |
| Sellar region mass, disease, radiation or trauma |
| Use of street drugs |
| Liver disease |
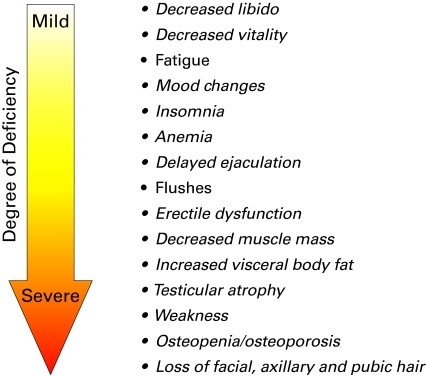
Beyond specific conditions, men may present with symptoms suggestive of TDS, including fatigue, insomnia, decreased libido, reduced vitality, mood changes, and erectile dysfunction. A comprehensive medical history and physical examination can reveal other clinical manifestations consistent with the severity of testosterone deficiency. These symptoms may occur individually or in combination.
Screening questionnaires, such as the Androgen Deficiency in Aging Males (ADAM) questionnaire, can be valuable tools for documenting patient history and symptoms. The ADAM questionnaire is widely used and has been assessed for its correlation with serum testosterone levels. While demonstrating high sensitivity in identifying men with biochemically low testosterone, its specificity is less robust. This means the ADAM questionnaire is effective at detecting TDS but may also flag some men who do not have the condition, highlighting the need for further biochemical evaluation for accurate TDS diagnosis.
TDS Diagnosis: Biochemical Evaluation and Confirmation
Patients exhibiting symptoms suggestive of TDS require biochemical evaluation of serum testosterone levels to confirm the diagnosis. It’s crucial to consider the circadian rhythm’s influence on testosterone, with levels typically higher in the morning. Therefore, blood samples for testosterone testing should ideally be drawn between 7 and 11 am to ensure accurate results for TDS diagnosis.
Testosterone in the blood circulates in both free and bound forms. The majority is bound to sex hormone-binding globulin (SHBG) or albumin. While SHBG binding is tight, albumin binding is weak. Bioavailable testosterone, encompassing both albumin-bound and free testosterone, is the fraction accessible to tissues and responsible for testosterone’s actions. SHBG-bound testosterone is biologically inactive. Generally, the severity of TDS symptoms correlates with the degree of testosterone deficiency.
While measured bioavailable testosterone is considered the gold standard for biochemical assessment in TDS diagnosis, it may not always be readily available or affordable. In such cases, calculated free testosterone (cFT), calculated bioavailable testosterone (cBT), or total testosterone (TT) are acceptable alternatives. Online calculators for cFT and cBT are available, such as the one provided by the International Society for the Study of the Aging Male (ISSAM). These calculated methods correlate well with laboratory-measured free testosterone, but limitations exist. Variability in SHBG level measurements across different assays and alterations in SHBG binding characteristics due to aging and illness can affect accuracy.
Due to equipment standardization challenges and interlaboratory variability, definitive cut-off values for normal testosterone levels are not universally defined. Instead, it’s recommended that clinicians consistently utilize the same local laboratories and become familiar with the accuracy, precision, and normal ranges specific to the assays offered in their community for reliable TDS diagnosis.
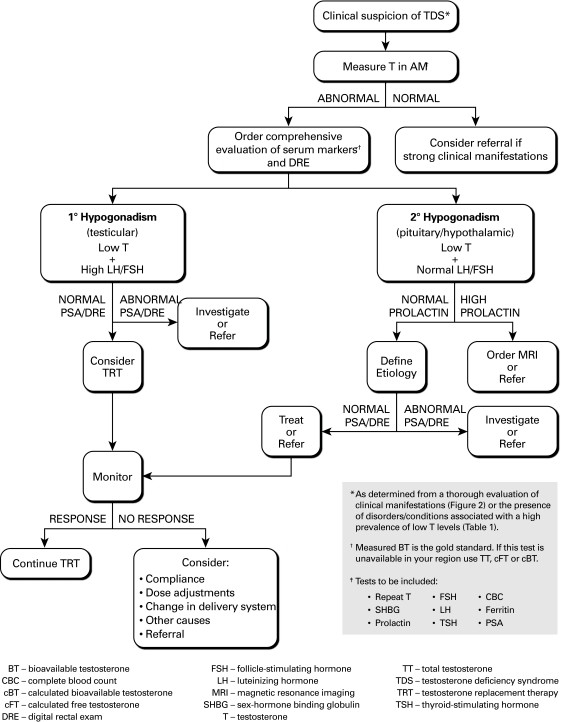
Significant intraindividual weekly variability in testosterone levels can occur in some men, and temporary testosterone levels below the normal range can be observed even in healthy young men. Therefore, abnormal (low or borderline) testosterone levels necessitate confirmation with a repeat testosterone measurement, along with assessments of SHBG and serum luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin. In younger men, chronically elevated LH and FSH with low testosterone definitively indicate primary (testicular) hypogonadism. However, these diagnostic criteria are less clear-cut in older men. Secondary hypogonadism (hypothalamic-pituitary) is characterized by low testosterone with normal LH and FSH levels. Often, a combination of primary and secondary factors contributes to TDS.
For a conclusive TDS diagnosis, it’s essential to rule out other clinical conditions that can mimic TDS symptoms. Conditions like depression or hypothyroidism share symptoms with TDS. Hypothyroidism can be excluded by measuring thyroid-stimulating hormone (TSH). Prolactin and ferritin levels can rule out hyperprolactinemia and hemochromatosis, respectively. Referral to a specialist should be considered for patients with elevated prolactin levels. To exclude pituitary/hypothalamic lesions, magnetic resonance imaging (MRI) is recommended in patients with very low testosterone and persistent hyperprolactinemia, or when prolactin levels are more than twice the upper normal limit.
Treatment of TDS: Addressing Testosterone Deficiency
Testosterone replacement therapy (TRT) is recommended for patients diagnosed with TDS. The primary goals of TRT are to alleviate symptoms and restore physiological testosterone levels. Various safe and effective testosterone formulations are available, each with different delivery mechanisms. Approved formulations in Canada include injectables, oral medications, and transdermal agents.
Common testosterone formulations available in Canada:
| Generic name | Trade name | Dosage |
|---|---|---|
| Injectables | ||
| Testosterone cypionate | Depo-testosterone | 200–400 mg every 2 weeks |
| Testosterone enanthate | Delatestryl | 100–400 mg every 1–4 weeks |
| Oral Medication | ||
| Testosterone undecanoate | Andriol pms-Testosterone | Initial dose of 120–160 mg per day in 2 divided doses |
| Transdermals | ||
| Testosterone patch | Androderm | 2.5 or 5 mg per day |
| Testosterone gels | AndroGel Testim | 5–10 g of gel per day |
Injectable testosterone formulations, such as testosterone cypionate (Depo-Testosterone) and testosterone enanthate (Delatestryl), are cost-effective, efficient, and long-acting. However, they can lead to supraphysiologic testosterone levels initially, followed by a waning effect that may cause symptom recurrence towards the end of the treatment cycle.
Oral testosterone undecanoate (Andriol or pms-Testosterone) offers convenience but may result in supraphysiologic dihydrotestosterone levels. Absorption can be inconsistent and requires consumption with a high-fat meal, potentially leading to variable responses.
Transdermal testosterone products, including gels (AndroGel and Testim) and patches (Androderm), provide consistent testosterone levels. Gels, applied daily, have minimal side effects, mainly mild skin reactions. Patches are similarly effective but may cause more noticeable skin reactions and be more visible. When using gels, patients should take precautions to avoid secondary exposure through skin contact.
The choice of testosterone formulation should be a collaborative decision between physician and patient, considering factors summarized by the ASTEP acronym: availability, safety, tolerability, efficacy, and patient preference. Physicians must be familiar with the follow-up requirements and potential adverse events associated with each formulation.
Benefits of TRT: Beyond TDS Diagnosis
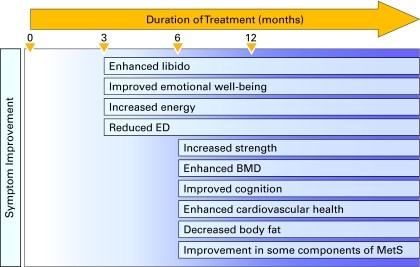
Testosterone replacement therapy offers numerous benefits beyond addressing TDS symptoms, potentially improving overall health and even survival. Clinical studies have demonstrated that TRT can enhance muscle strength, sexual desire, energy levels, emotional well-being, and cognitive function. TRT also increases bone mineral density in men with low testosterone, reduces body fat, improves glycemic control in diabetic patients, and may positively impact components of metabolic syndrome. Men with severe hypogonadism often experience significant improvement in erectile dysfunction with TRT. Emerging evidence suggests potential cardiovascular benefits of testosterone therapy in hypogonadal men.
Contraindications to TRT: Safety Considerations Post TDS Diagnosis
Prior to initiating TRT, patients must be evaluated for contraindications and conditions that could be worsened by treatment. Testosterone therapy is strictly contraindicated in men with breast or prostate cancer. Prostate-specific antigen (PSA) level measurement and a digital rectal examination (DRE) are recommended before starting TRT. Abnormal PSA or DRE findings necessitate referral to a urologist for further evaluation before considering TRT.
TRT can also exacerbate conditions like erythrocytosis, untreated obstructive sleep apnea, and severe congestive heart failure. Erythrocytosis can be detected via a complete blood count (CBC). TRT is not advised for men desiring biological fatherhood as it can reduce sperm production. These conditions should be addressed before TRT initiation.
Alternative Treatments to TRT: Complementary Approaches After TDS Diagnosis
In some cases, managing TDS symptoms may involve addressing underlying causes, medication adjustments, or lifestyle modifications. Treating sleep apnea, weight loss, and discontinuing opioid medications can improve TDS manifestations. However, adherence to lifestyle changes like diet and exercise can be challenging, making TRT a practical option for many.
Common alternative/complementary approaches to testosterone replacement therapy:
| Approach | Anticipated outcomes |
|---|---|
| Diet and exercise | Healthy weight reductionImproved muscle strengthEnhanced emotional well-being |
| Bisphosphonates | Increased bone mineral density |
| Antidepressants | Enhanced emotional well-being |
| Continuous positive airway pressure | Treatment of sleep apnea |
| Phosphodiesterase-5 inhibitors | Improved erectile function |
| Discontinuation of opioids | Improvement in multiple symptoms of hypogonadism |
Monitoring TRT: Long-Term Management Post TDS Diagnosis
Regular monitoring is essential to assess symptom response and monitor blood parameters in patients undergoing TRT. Monitoring should occur every 3 to 6 months during the first year and annually thereafter if the patient remains stable. Symptom improvement may be observed within 3 to 6 months of TRT initiation, while other symptoms may take longer to resolve. Lack of response may indicate adherence issues, malabsorption, inadequate dosage, unsuitable formulation, or symptoms unrelated to TDS. Persistent lack of response may warrant specialist referral. Combination therapy with testosterone and phosphodiesterase-5 inhibitors (PDE5i) may be beneficial for ED patients who do not respond to TRT alone. Similarly, ED patients who fail PDE5i monotherapy should be screened for TDS and may benefit from adding testosterone to their treatment.
At each follow-up appointment, clinical response, adverse events, serum testosterone levels, hemoglobin, and hematocrit should be evaluated. Prostate health should be monitored via PSA and DRE. Referral is necessary for suspicious DRE findings or increasing PSA levels. Elevated hematocrit, indicative of erythrocytosis, a potential side effect of TRT, particularly with depot formulations, may require dose adjustments or a change in formulation to maintain hematological values within the normal range.
Conclusion: Key Takeaways on TDS Diagnosis and Management
This guide emphasizes critical aspects of TDS:
- TDS prevalence is significant in men over 40, yet a small proportion receive adequate treatment, highlighting gaps in TDS diagnosis and care.
- TDS is a significant health issue due to its links with diabetes, cardiovascular disease, osteoporosis, and reduced quality of life and life expectancy.
- TDS diagnosis requires both clinical symptoms and laboratory confirmation of abnormal testosterone levels.
- Confirmed TDS can be managed through lifestyle modifications and appropriate use of injectable, oral, or transdermal testosterone preparations.
- Regular monitoring of symptoms and TRT safety is crucial, involving physical exams, serum analysis, and DRE.
Appendix A. ADAM Questionnaire
| A positive answer represents Yes to questions 1 or 7 or any 3 other questions |
|---|
| YES |
| □ |
| □ |
| □ |
| □ |
| □ |
| □ |
| □ |
| □ |
| □ |
| □ |
*Morley et al. (6).