Introduction
Myocardial Perfusion Imaging (MPI) has been a cornerstone in the diagnosis and risk assessment of patients with coronary artery disease (CAD) for over three decades.1 Its proven diagnostic and prognostic capabilities, coupled with its ability to offer insights into cardiac function through perfusion and functional parameter analysis, solidify its importance.2,3 Among these functional parameters, transient ischemic dilatation (TID) stands out as a significant indicator. Studies have consistently validated TID as a marker for both severe, extensive coronary artery disease and a predictor of adverse cardiac outcomes.1–3 Understanding Tid Diagnosis is crucial for effective risk stratification and patient management in cardiology.
The underlying mechanisms of ischemic left ventricular (LV) dilatation are still being researched, with subendocardial ischemia being the most widely accepted theory.4,5 Alternative theories propose that TID represents ischemia-induced physical LV dilatation following stress.6,7 However, it’s important to note that transient ischemic dilatation can also occur in patients with seemingly normal perfusion and without significant epicardial coronary artery disease. This phenomenon has been observed in conditions such as hypertrophic cardiomyopathy8 and hypertensive heart disease with left ventricular hypertrophy.9 These observations bring to light the ongoing debate surrounding the true diagnostic accuracy of TID in MPI and the need for a more precisely defined threshold for its identification.
This study undertakes a comprehensive meta-analysis to evaluate the diagnostic performance of TID in comparison to anatomical coronary artery assessments. Furthermore, it systematically reviews the prognostic significance of TID. Both aspects of this research include data from patients undergoing MPI with either exercise or pharmacological stress, providing a broad perspective on tid diagnosis utility.
Methods
To conduct this research, a systematic search was performed across MEDLINE, EMBASE, and COCHRANE databases. The search focused on English language studies investigating the diagnostic and/or prognostic accuracy of transient ischemic dilatation in myocardial perfusion imaging. Keywords employed in the search strategy included: “transient ischemic dilation,” “transient ischaemic dilation,” “left ventricular dilation,” “transient dilation,” “SPECT,” “single photon tomography,” “CT single photon,” “myocardial perfusion imaging,” and “myocardial scintigraphy.” This comprehensive search strategy aimed to capture all relevant studies related to tid diagnosis.
Two independent investigators (MA, HE) meticulously reviewed the identified studies and extracted pertinent data. This data included patient demographics, the specific radiotracer used, the stress modality employed, and findings from coronary angiography. In instances where data was missing for the meta-analysis or discrepancies were noted, efforts were made to contact the original study authors to obtain the necessary information and ensure accuracy in the assessment of tid diagnosis. Studies were excluded based on the following criteria: (a) lack of coronary arterial anatomic assessment (either invasive coronary angiogram or coronary CT angiography) for comparison, or (b) absence of clear documentation detailing the method used to calculate the ratio defining TID positivity.
The studies selected for inclusion and the extracted data underwent further review by a third investigator (DSL) to confirm eligibility and accuracy. Methodological quality was assessed using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS 2) tool,10 which evaluates the risk of bias and concerns regarding applicability in diagnostic accuracy studies for systematic reviews and meta-analyses. QUADAS 2 encompasses four key domains: patient selection, index test, reference standard, and flow and timing, providing a structured approach to assess the robustness of studies related to tid diagnosis.
Statistical Analysis
Data extraction was structured to create 2×2 tables, enabling the calculation of sensitivity and specificity for each study. To account for the correlation between TID in MPI and anatomic disease severity, sensitivity and specificity estimates were pooled using a bivariate random effects model, a method recommended by the Cochrane Diagnostic Test Accuracy Working Group.11 This approach is crucial for robust meta-analysis in tid diagnosis research.
The bivariate model further facilitated the construction of a hierarchical summary receiver operator curve (ROC). Heterogeneity between diagnostic studies was assessed using the P value, recognizing the limitations of the 2 statistic as a measure of heterogeneity in this context.11 Univariate meta-regression was employed to evaluate the significance of key covariates potentially influencing the diagnostic accuracy of the test for tid diagnosis. Due to the limited number of studies, multivariate meta-regression was deemed statistically underpowered and not performed.
All statistical analyses were performed using STATA/SE, version 12.0 (Stata Corp LP, College Station, Texas, USA).
Results
Summary of Studies Examining TID
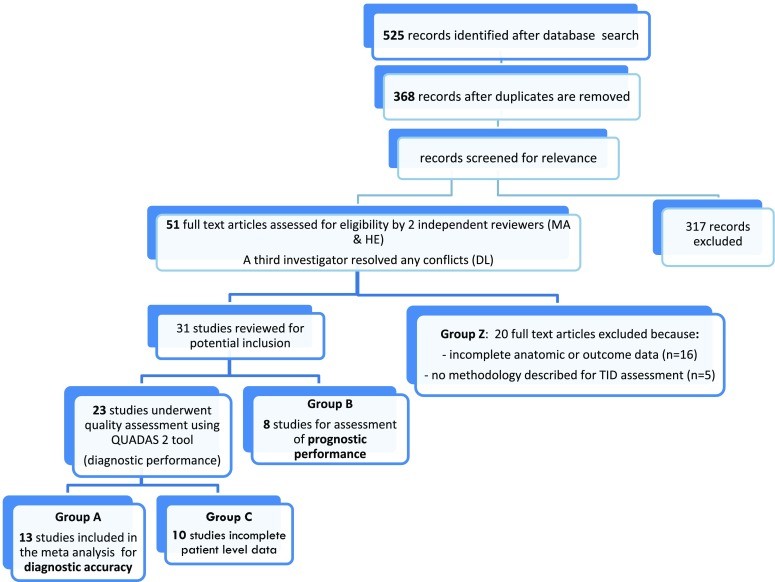
The initial database search yielded 525 citations, which were reduced to 368 articles after removing duplicates. Review of titles and abstracts led to the exclusion of 317 articles deemed irrelevant to the study’s purpose. This rigorous screening process ensured focus on studies directly related to tid diagnosis.
From the remaining 51 articles, 20 were further excluded for various reasons: (a) no evaluation of coronary anatomy (invasive coronary angiogram or CT angiography) for diagnostic studies [n=11], (b) inclusion of only patients with left ventricular dysfunction and fixed perfusion defects for prognostic studies [n=1], (c) lack of clear documentation of the method used for TID ratio calculation or reliance on visual assessment of TID [n=5], (d) incomplete data [n=2], and (e) duplicate data [n=1], as detailed in Figure 1 (Group Z). Online Table A lists these excluded studies. The final analysis included 31 studies, of which 23 assessed TID from a diagnostic standpoint. Among these, 13 studies were included in the quantitative meta-analysis (Figure 1, Group A), while 10 studies were not quantitatively synthesized due to aggregated patient-level data that prevented separation into TID positive/negative or severe/non-severe CAD categories (Group C). The quantitative meta-analysis comprised a total of 2037 patients in the diagnostic evaluation and 9003 patients in the prognostic evaluation (Figure 1). Additionally, 8 studies examining the prognostic significance of TID, lacking patient-level data, were incorporated into a narrative synthesis (Group B).
Figure 1.
Figure 1: This flowchart illustrates the study selection process for the meta-analysis of tid diagnosis, detailing the initial search results, screening stages, and reasons for exclusion, ultimately leading to the final set of studies included in the diagnostic and prognostic analyses.
Studies of Diagnostic Performance
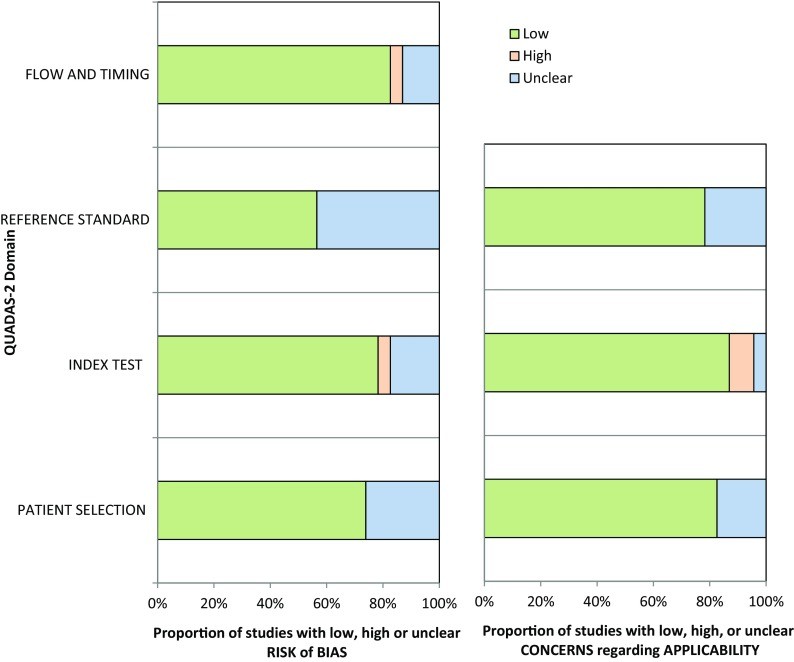
Table 1 summarizes the characteristics of the 13 studies included in the quantitative meta-analysis, while Online Table B details the 10 non-quantitatively analyzed studies. Table 2 outlines the definitions of severe and extensive CAD and the software used for TID evaluation across studies related to tid diagnosis. Technetium-99m was the most prevalent radiotracer (8 studies), followed by Tl-201 (2 studies), dual isotope scanning (2 studies), and Rb-82 (1 study). Coronary angiography was performed in 73% of patients. Stress modalities included exercise (4 studies), pharmacologic stress (6 studies), and a combination of both (3 studies). QUADAS 2 assessment revealed that most studies had a low risk of bias and applicability concerns (Figure 2).
Table 1.
Studies included in the meta-analysis of diagnostic performance of TID
| Study | Stress/tracer | TID ratio (quant or qual) | # SPECT vs coronary angiography or CTA | Cardiac risk factors | Cardiac characteristics |
|---|---|---|---|---|---|
| Xu et al28 62.4 ± 10.0 years62% men | ExerciseTc 99m | 1.19 (quant) | SPECT: 547 pts, angiography or CTA: 215 pts | Smoking 15%, HTN 59%, DM 18%, Dyslipidemia 50% | Angina 17% |
| Kinoshita et al29 63.1 ± 7.8 years87% men | ExerciseTc 99m | 1.012 (quant) | SPECT: 75 pts, angiography or CTA: 55 pts | N/A | N/A |
| Petretta et al30 63 ± 9 years75% men | Ex and PharmTc 99m | 1.19 (quant) | SPECT: 692 pts, angiography or CTA: 242 pts | Smoking 47%, HTN 88%, DM 100%, Dyslipidemia 75% | Angina 31%, MI 16% |
| Weis et al1 58 years78% men | ExerciseTl 201 | 1.12 (quant and qual) | SPECT: 89 pts, angiography or CTA: 89 pts | N/A | N/A |
| Chouraqui et al6 67 ± 3 years47% men | PharmTl 201 | 1.12 (quant) | SPECT: 73 pts, angiography or CTA: 73 pts | N/A | MI 33% |
| Emmett et al31 68 ± 10 years64% men | AdenosineTc 99m | 1.19 (quant) | SPECT: 175 pts, angiography or CTA: 55 pts | HTN 82%, DM 81% | N/A |
| Fallahi et al18 56 ± 11 years23% men | PharmTc 99m | 1.17 (quant) | SPECT: 86 pts, angiography or CTA: 38 pts | Smoking 17%, HTN 55%, Dyslipidemia 36% | N/A |
| Emmett et al32 72 ± 8 years,47% men | Ex and PharmTc 99m | 1.22 (quant and qual) | SPECT: 103 pts, angiography or CTA: 103 pts | DM 58% | N/A |
| Marcassa et al5 61 ± 8 years% men N/A | Ex and PharmTc 99m | 1.24 (quant) | SPECT: 234 pts, angiography or CTA: 186 pts | HTN 33%, DM 5%, Dyslipidemia 42% | Angina 47%, MI 63% |
| Rischpler et al27 64 ± 11 years58% men | PharmRb 82 | 1.13 (quant) | SPECT: 265 pts, angiography or CTA: 81 pts | Smoking 26%, HTN 84%, DM 37%, Dyslipidemia 58% | MI 26%, PCI 42% |
| Mazzanti et al33 66 ± 11 years% men N/A | ExerciseDual | 1.22 (quant) | SPECT: 228 pts, angiography or CTA: 174 pts | N/A | MI 35%, CABG 33% |
| Abidov et al34 69 ± 11.4 years54% men | PharmDual | 1.36 (quant) | SPECT: 356 pts, angiography or CTA: 179 pts | HTN 70%, DM 31% | Angina 48% |
| Golzar et al12 62 ± 1350% men (validation group) | PharmTc 99m | 1.31 (quant) | SPECT: 647 pts, angiography or CTA: 547 pts | HTN 86%, Dyslipidemia 63% | Chest pain 54% |
Open in a new tab
Table 1: This table presents the key characteristics of studies included in the meta-analysis focusing on the diagnostic performance of TID. It details stress modalities, radiotracers, TID ratio definitions, patient demographics, cardiac risk factors, and cardiac characteristics, offering a comprehensive overview of the data used to evaluate tid diagnosis.
Table 2.
Definition of CAD severity and software used for TID calculation
| Study | Definition of severe and extensive CAD | Software used for TID calculation |
|---|---|---|
| Xu et al28 | ≥70% LM or proximal LAD or ≥90% of ≥2 coronary vessels | Cedars Sinai QPS and QGS software |
| Kinoshita et al29 | ≥75% reduction of luminal diameter of ≥2 coronary vessels | GMS-550 A Toshiba workstationFormula used for calculation (Mean A EX/Mean A rest) |
| Petretta et al30 | ≥70% proximal LAD or ≥90% of ≥2 coronary vessels | Cedars Sinai software |
| Weis et al1 | ≥90% of ≥2 coronary vessels | Manual calculation form the anterior view planar images |
| Chouraqui et al6 | ≥90% of 3 coronary vessels | Manual calculation form the anterior view planar images |
| Emmett et al31 | ≥90% of LAD or ≥90% of ≥2 coronary vessels | Emory Cardiac toolbox |
| Fallahi et al18 | Coronary artery index Gensini score used | Cedars Sinai Auto Quant software package |
| Emmett et al32 | ≥90% of LAD or ≥90% of ≥2 coronary vessels | Emory Cardiac toolbox |
| Marcassa et al5 | >50% diameter stenosis in major pericardial vessel modified Gensini score | Not specified |
| Rischpler et al27 | Obstructive CAD (Cath/CTA) severity not specified | Card IQ Physio (GE healthcare) |
| Mazzanti et al33 | ≥90% of proximal LAD or ≥90% of ≥2 coronary vessels | Cedars Sinai QPS and QGS software |
| Abidov et al34 | ≥90% of proximal LAD or ≥90% ≥2 vessel disease | Cedars Sinai QPS software |
| Golzar et al12 | ≥70% LM or proximal LAD or ≥90% of ≥2 coronary vessels | 4DM-SPECT version 5.1 |
Table 2: This table details the definitions of severe and extensive coronary artery disease (CAD) used across the included studies and the software employed for TID calculation. Variations in CAD definition and calculation methods highlight the need for standardized approaches in tid diagnosis research and clinical practice.
Figure 2.
Figure 2: This graph summarizes the quality assessment of the studies using the QUADAS-2 tool, indicating the risk of bias and concerns regarding applicability across different domains. The generally low risk scores support the reliability of the included studies in evaluating tid diagnosis.
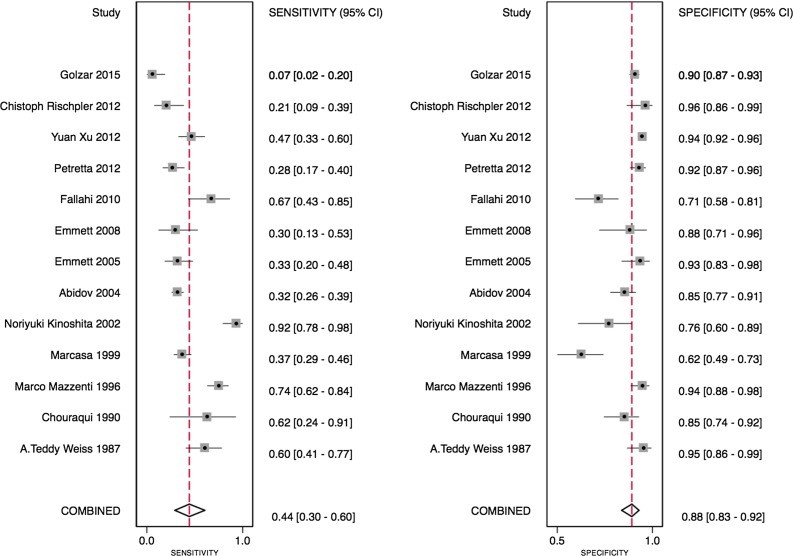
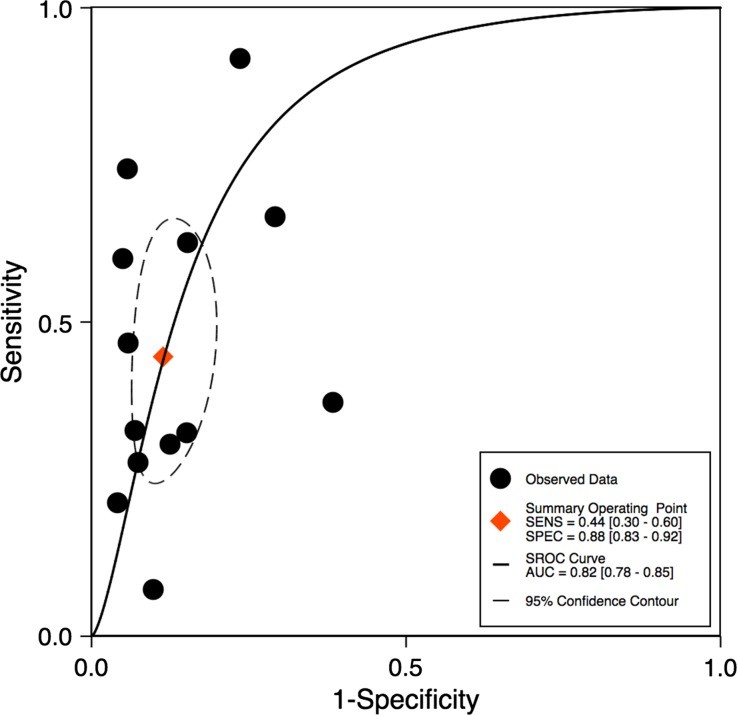
The TID ratio cutoff for defining TID presence varied from 1.13 to 1.38, influenced by the radiotracer used. Utilizing the TID ratios defined by individual study authors, the sensitivity of TID for detecting extensive or severe CAD ranged from 21% to 62.5% (Table 3). Notably, one study demonstrated a particularly low sensitivity of 7%.12 Specificities were generally higher and less variable, ranging from 77% to 98% (Table 3). Bivariate analysis of the 13 studies with complete statistical data revealed a pooled sensitivity of 44% (95% confidence interval [CI] 30%-60%) and a pooled specificity of 88% (95% CI 83%-92%), as illustrated in Figure 3. The pooled area under the ROC curve was 0.82 (0.78-0.85) for all studies (Figure 4), indicating good overall diagnostic accuracy of tid diagnosis.
Table 3.
Diagnostic performance of TID in meta-analyzed studies
| Study | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|
| Xu et al28 | 56 | 90 | 56 | 90 |
| Kinoshita et al29 | 91.4 | 76.9 | 79 | 90 |
| Petretta et al30 | 27.5 | 92.9 | 59.3 | 76 |
| Weis et al1 | 60 | 95 | 85 | 82 |
| Chouraqui et al6 | 62.5 | 85 | – | – |
| Emmett et al31 | 30.4 | 87.5 | 63.6 | 63.6 |
| Fallahi et al18 | 66.7 | 70.8 | – | – |
| Emmett et al32 | 30 | 93 | 79 | 63 |
| Marcassa et al5 | 37 | 61 | 64 | 34 |
| Rischpler et al27 | 21 | 95.8 | 77 | 63 |
| Mazzanti et al33 | 77 | 92 | – | – |
| Abidov et al34 | 73 | 88 | – | – |
| Golzar et al12 | 7 | 98 | – | – |
Table 3: This table summarizes the diagnostic performance metrics of TID from the meta-analyzed studies, including sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The variability in sensitivity and generally high specificity underscore the complex nature of tid diagnosis and its interpretation.
Figure 3.
Figure 3: This forest plot visually represents the sensitivity and specificity of individual studies included in the diagnostic meta-analysis for tid diagnosis, along with their confidence intervals, and the pooled estimates, providing a clear comparison of study results and overall diagnostic performance.
Figure 4.
Figure 4: This ROC curve illustrates the overall diagnostic accuracy of tid diagnosis based on the pooled data from all included studies, with the area under the curve (AUC) indicating the discriminative ability of TID in identifying severe coronary artery disease.
Subgroup Analyses for Diagnostic Performance of TID
Subgroup analysis of technetium studies showed a pooled sensitivity of 42% (95% CI 23%-63%) and specificity of 86% (95% CI 78%-92%), consistent with the overall results (Figure 5). Subgroup analyses also indicated that exercise stress studies had a significantly higher pooled area under the ROC curve (AUC 0.92 vs 0.78, P < 0.05). In studies using qualitative TID assessment, pooled sensitivity was 46% (95% CI 38%-54%) and specificity was 88% (95% CI 79%-93%).
Figure 5.
Figure 5: This figure presents ROC curves for subgroup analyses, comparing diagnostic accuracy of tid diagnosis across different tracers and stress modalities, highlighting potential variations in performance based on these factors.
For technetium studies, the TID ratio range was 1.13-1.31. Sensitivity in these studies ranged from 34% to 56%, and specificity from 77% to 98% for severe CAD detection. Overall sensitivity and specificity were similar for both exercise and pharmacologic technetium studies. Univariate meta-regression showed that the TID ratio cutoff had minimal effect on specificity (P = 0.99), but higher TID ratios were associated with decreased sensitivity (P < 0.05).
Studies of Prognostic Performance
Table 4 summarizes studies evaluating TID as a predictor of cardiac events. Annualized rates of cardiac death or MI ranged from 0.2% to 1% in patients without TID, 2% to 5% in those with TID and normal perfusion, and 5% to 6% in patients with TID and ischemia, CAD, or diabetes. De Winter et al. 13 reported TID as a significant predictor of all-cause mortality even after adjusting for resting heart rate, beta-blocker use, summed rest score, and resting LV ejection fraction, emphasizing the independent prognostic value of tid diagnosis.
Table 4.
Studies included in the systematic review of prognosis
| Study | Demographics and follow-up | TID ratio (quant or qual), n(%) of pts with TID | Annualized cardiac outcomes |
|---|---|---|---|
| Abidov et al17 66.1 ± 12.8 years50% menEx and PharmDual | Primary study cohort: 1560 pts with normal perfusion;Secondary cohort: 2037 pts with normal or near-normal perfusion:2.3 ± 0.7 years | 1.21 (quant)n = 390 (25%) | Outcome: cardiac death, MI, or coronary revascularizationNormal perfusion (SSS = 0):(a) TID present: 2.4%·year−1;(b) No TID (i.e., lowest TID quartile): 0.6%·year−1 Normal/near-normal perfusion (SSS 0-3):(a) TID present: 2.2%·year−1;(b) Other 3 TID quartiles: 1.0%·year−1 |
| Petretta et al14 64 ± 8 years67% menEx and PharmTc 99m | 672 pts with DM2+ years | 1.19 (quant)n = 97 (14%) | Outcome: cardiac death or MINo ischemia + LVEF > 45%:(a) TID present: 4.9%·year−1 (b) No TID: 0.21%·year−1 Ischemia present + LVEF > 45%:(a) TID present: 5.4%·year−1 (b) No TID: 1.9%·year−1 Ischemia + LVEF ≤ 45%:(a) TID present: 7.2%·year−1 (b) No TID: 3.8%·year−1 |
| Doukky et al19 58 ± 12 years52% menEx and PharmTc 99m | 1236 pts with normal perfusion27 ± 9 (clinical events)37 ± 8 months (mortality) | 1.16 (Ex)1.22 (Pharm)(quant)n = 76 (6%) | Outcome: cardiac death or MIOverall:(a) TID present: 2.4%·year−1 (b) No TID: 0.4%·year−1 DM or CAD:(a) TID present: 5.9%·year−1 (b) No TID: 0.2%·year−1 |
| Uz zaman et al15 56 ± 9 years67% menEx and PharmTc 99m | 2689 pts in a single-center18 ± 4 months | 1.22 (quant)n = 189 (7%) | Outcome: fatal MI(a) TID + not revascularized: 3.3%·year−1 (b) TID + revascularized: 1.6%·year−1 (c) No TID: not reportedOutcome: non-fatal MI(a) TID + not revascularized: 14.2%·year−1 (b) TID + revascularized: 3.3%·year−1 (c) No TID: not reported |
| Lette et al16 61 ± 10 years55% menPharmTl 201 | 510 total pts;Prognostic cohort: 187 undergoing non-cardiac surgery12 months | 1.15 (quant)n = 45 (9%) | Outcome: cardiac death or MI(a) TID present: 58%·year−1 (b) Reversible defect: 19%·year−1 (c) Normal: 2%·year−1 |
| Lette et al35 62 years57% menPharmTl 201 | 753 pts16 months | 1.15 (quant)n = 41 (5%) | Outcome: cardiac death or MI(a) TID present: 47.4%·year−1 (b) Reversible defect: 15.8%·year−1 (c) Normal: 2.0%·year−1 |
| Thomas et al36 65 ± 12 years62% menEx and PharmTc 99m and Dual | 1612 pts24 ± 7 months | Qualitativen = 371 (23%) | Outcome: cardiac death or MITechnetium:(a) TID present: 6%·year−1 (b) No TID: 1.5%·year−1 Dual isotope:(a) TID present: 3.8%·year−1 (b) No TID: 0.5%·year−1 |
| De winter et al13 78 (IQR: 76-80) years54% menEx and PharmTc 99m | 294 pts aged ≥ 75 years old26 months | 1.005 (quant)n = 147 | Outcome: all-cause death(a) TID present: 9.7%·year−1 (b) No TID: 5.0%·year−1 |
Table 4: This table provides a summary of studies included in the systematic review of prognosis, examining the prognostic value of TID. It details patient demographics, follow-up duration, TID ratio definitions, and annualized cardiac outcomes, illustrating the association between tid diagnosis and future cardiac events.
Patient heterogeneity was observed across prognostic studies. In a study by Petretta et al.14 on diabetic patients with TID and ischemia, the annualized rate of cardiac death or non-fatal MI was 7.2% with post-stress LVEF ≤45% and 5.4% with LVEF >45%. Uz zaman et al.15 found significantly increased rates of fatal and non-fatal MI in patients with TID who were not revascularized compared to those who underwent revascularization. Lette et al.16 reported high postoperative cardiac event rates in patients undergoing MPI before non-cardiac surgery: 58% with TID, 19% with reversible perfusion defects but no TID, and 2% with normal scans, with most events occurring within 4 months postoperatively, highlighting the critical prognostic implications of tid diagnosis in perioperative risk assessment.
Special Consideration of TID in Patients with Otherwise Normal Perfusion Scans
Abidov et al.17 demonstrated TID as an independent prognostic marker for cardiac events even in patients with normal or near-normal MPI. Patients in the highest TID quartile (mean TID ratio 1.35 ± 0.14) were older and more likely to be diabetic. The prognostic impact of TID in the context of normal myocardial perfusion was modified by CAD or diabetes presence,18 with increased risk of cardiac death or MI reported in these patient groups. Doukky et al.19 and Petretta et al.14 further supported these findings in diabetic patients with normal post-stress LVEF and no ischemia, where annual event rates were significantly higher in those with TID compared to those without, emphasizing the importance of tid diagnosis even in seemingly low-risk MPI results.
Discussion
This meta-analysis reveals that transient ischemic dilatation is a specific, but not highly sensitive, marker for detecting severe and extensive CAD, with a pooled sensitivity of 44% and specificity of 88%. Subgroup analysis indicated a trend towards higher sensitivity with exercise stress compared to pharmacologic stress, while specificity remained consistent. Prognostic studies consistently showed elevated cardiac risk in patients with TID, despite variations in studied populations, underscoring the robust prognostic value of tid diagnosis.
The risk associated with TID was further amplified in patients with reduced post-stress LVEF (≤ 45%), with cardiac death or MI rates exceeding 7% annually. In patients with normal perfusion scans, TID was particularly prognostic in those with pre-existing CAD or diabetes mellitus, highlighting the nuanced interpretation required for tid diagnosis in different clinical contexts.
While numerous studies have explored diagnostic test performance, meta-analytic approaches focusing on specific high-risk markers like TID are less common. Existing meta-analyses primarily address broader imaging modalities like stress echocardiography,20 cardiac PET,21 and coronary CT angiography,22,23. This study fills a critical gap by providing the first meta-analysis, to our knowledge, specifically evaluating the quantitative diagnostic performance of TID for severe CAD detection and its prognostic performance for predicting cardiac outcomes.
Mechanistic insights may explain TID’s high specificity and lower sensitivity. Subendocardial ischemia, leading to reduced radiotracer uptake in the endocardium and apparent LV dilatation, is a proposed mechanism.4,[5](#CR5] Alternatively, TID might represent post-stress LV dilatation due to ventricular dysfunction.24 These mechanisms suggest that TID is more likely to manifest in severe CAD, contributing to its high specificity but potentially limiting sensitivity in less severe or extensive disease. Furthermore, TID can occur in conditions like hypertensive heart disease and hypertrophic cardiomyopathy, and in certain 2-day MPI protocols with normal epicardial coronary vessels,8,9 which can also affect its diagnostic performance in the context of tid diagnosis.
Transient ischemic dilatation in myocardial perfusion imaging has been recognized as a significant diagnostic and prognostic marker for severe and extensive CAD, yet its clinical utility varies across literature. This meta-analysis addresses critical aspects by: (a) examining the range of TID ratio cutoffs, (b) systematically reviewing pooled diagnostic performance, and (c) evaluating prognostic value. Our findings validate TID’s role as a high-risk marker in MPI, indicating stress-induced myocardial ischemia and predicting future cardiac events. Based on these results, we propose a modified algorithm approach25 for patients with TID. The co-occurrence of TID and high-risk MPI strongly suggests considering invasive coronary angiography.[25](#CR25] Given TID’s high specificity, in cases of TID with non-high risk MPI (SSS < 4), further risk stratification is warranted, especially in diabetic patients or those with known CAD, to guide management decisions for tid diagnosis. Finally, applying quantitative meta-analysis to evaluate other high-risk diagnostic markers within imaging modalities,26 could prove valuable in future cardiac imaging research.
While meta-analyses are powerful tools, inherent limitations exist, such as heterogeneity in patient populations, techniques, and diagnostic cutoffs across studies. Although higher TID ratios impacted sensitivity, meta-regression did not identify other clinical variables significantly affecting effect estimates. The limited number of studies and inter-study differences in stress modality and tracer might have underrepresented the influence of these factors. Most studies defined angiographic CAD severity using percent stenosis, except for Rischpler et al.[27](#CR27]. We included Rischpler et al. due to high rates of prior cardiac history and its unique use of Rb PET.[27](#CR27], but sensitivity analysis excluding this study showed no significant change in diagnostic performance, reinforcing the robustness of our findings for tid diagnosis.
New Knowledge Gained
This meta-analysis demonstrates that TID exhibits a high pooled area under the ROC curve for detecting severe and extensive CAD. While sensitivity is moderate, the specificity of TID is notably high in identifying severe and extensive CAD.
The risk of cardiac death or MI is elevated in patients with TID and normal perfusion, particularly among those with diabetes mellitus, pre-existing CAD, or ischemia. This risk appears to escalate further in patients with reduced left ventricular ejection fraction, emphasizing the importance of considering these factors in tid diagnosis interpretation.
Conclusion
In conclusion, this meta-analysis confirms that transient ischemic dilatation during myocardial perfusion imaging is a specific diagnostic marker for severe and extensive coronary artery disease. TID serves as a significant indicator of poor prognosis, with risks markedly increased in patients with evidence of coronary disease or reduced stress LV ejection fraction. Even in diabetic patients with normal perfusion, the presence of TID significantly worsens prognosis. Therefore, transient ischemic dilatation should be recognized as a high-risk marker that can effectively guide clinical management strategies in patients with suspected or known coronary artery disease, making tid diagnosis a critical component of cardiac risk assessment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 137 kb) (137.3KB, docx)
Supplementary material 2 (PPTX 4413 kb) (4.3MB, pptx)
Acknowledgments
This research was supported by a Foundation Grant from the Canadian Institutes of Health Research (Grant
FDN 148446). Dr Lee is supported by a mid-career investigator award from the Heart and Stroke Foundation and is the Ted Rogers Chair in Heart Function Outcomes, a joint Hospital-University Chair of the University Health Network and the University of Toronto. The Institute for Clinical Evaluative Sciences (ICES) is supported in part by a grant from the Ontario Ministry of Health and Long-Term Care. The opinions, results, and conclusions are those of the authors and no endorsement by the Ministry of Health and Long-Term Care or by the Institute for Clinical Evaluative Sciences is intended or should be inferred.
Disclosures
The study authors have no financial conflicts of interest.
Note
This paper is dedicated to the memory of Dr. Michael Freeman (Dec 29, 1948–Sept 3, 2017).
Abbreviations
CABG Coronary artery bypass graft
CAD Coronary artery disease
CI Confidence interval
CT Computed tomography
DM Diabetes mellitus
LVEF Left ventricular ejection fraction
MI Myocardial infarction
PCI Percutaneous coronary intervention
ROC Receiver operating characteristic
TID Transient ischemic dilation
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12350-017-1040-7) contains supplementary material, which is available to authorized users.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
JNC thanks Erick Alexanderson MD, Carlos Guitar MD, and Diego Vences MD, UNAM, Mexico, for providing the Spanish abstract; Haipeng Tang MS, Zhixin Jiang MD, and Weihua Zhou PhD, for providing the Chinese abstract; and Jean-Luc Urbain, MD, PhD, CPE, Past President CANM, Chief Nuclear Medicine, Lebanon VAMC, PA, for providing the French abstract.
References
[References]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 137 kb) (137.3KB, docx)
Supplementary material 2 (PPTX 4413 kb) (4.3MB, pptx)