The landscape of obesity and cardiovascular disease treatment has recently shifted, particularly concerning Medicare coverage for medications like Wegovy and Ozempic. A pivotal decision by the Food and Drug Administration (FDA) has broadened the potential for Medicare to cover Wegovy, a drug initially approved for weight loss, and raises questions about the future of coverage for similar medications, including Ozempic. This article delves into the implications of this FDA decision, exploring what diagnoses might now qualify patients for coverage of these GLP-1 agonists under Medicare.
The FDA’s Game-Changing Approval for Wegovy
Wegovy (semaglutide), a notable anti-obesity medication, has received FDA approval for a significant new use: reducing the risk of serious cardiovascular events such as heart attacks and strokes in adults with established cardiovascular disease who are also overweight or obese. This expansion is critical because it moves Wegovy beyond being solely categorized as a weight-loss drug, recasting it as a medication that addresses serious health risks associated with cardiovascular conditions in overweight individuals.
This reclassification is crucial for Medicare because of existing legal restrictions. Medicare is generally prohibited from covering drugs specifically for weight loss. However, medications approved for other medically accepted indications can be covered. Semaglutide, under the brand name Ozempic, is already covered by Medicare for the treatment of type 2 diabetes. The FDA’s new approval for Wegovy, therefore, creates a pathway for Medicare Part D coverage, as it now serves a purpose beyond just obesity treatment in a specific patient group.
Medicare’s Response: Opening the Door for Wegovy Coverage
Following the FDA’s decision, the Centers for Medicare & Medicaid Services (CMS) quickly signaled a change in stance. CMS issued a memo clarifying that Medicare Part D plans can now include Wegovy on their formularies. This is because Wegovy now carries a “medically-accepted indication” that isn’t solely for an excluded category (i.e., weight loss). As Wegovy is an injectable medication for self-administration, coverage falls under Medicare Part D, which is for outpatient prescription drugs, managed by private plans, rather than Part B, which covers drugs administered by physicians.
Who Qualifies? Diagnoses and Eligibility for Wegovy Under Medicare
The new FDA indication targets individuals with established cardiovascular disease – meaning a history of heart attack, stroke, or peripheral arterial disease – who are also classified as obese or overweight. Analyzing 2020 Medicare data, it’s estimated that around 7% of Medicare beneficiaries, or 3.6 million people, fit this profile. This represents a significant portion of the 13.7 million Medicare beneficiaries diagnosed with obesity or overweight in 2020, suggesting that roughly 1 in 4 Medicare beneficiaries with obesity or overweight could potentially gain access to Wegovy coverage due to this expanded indication.
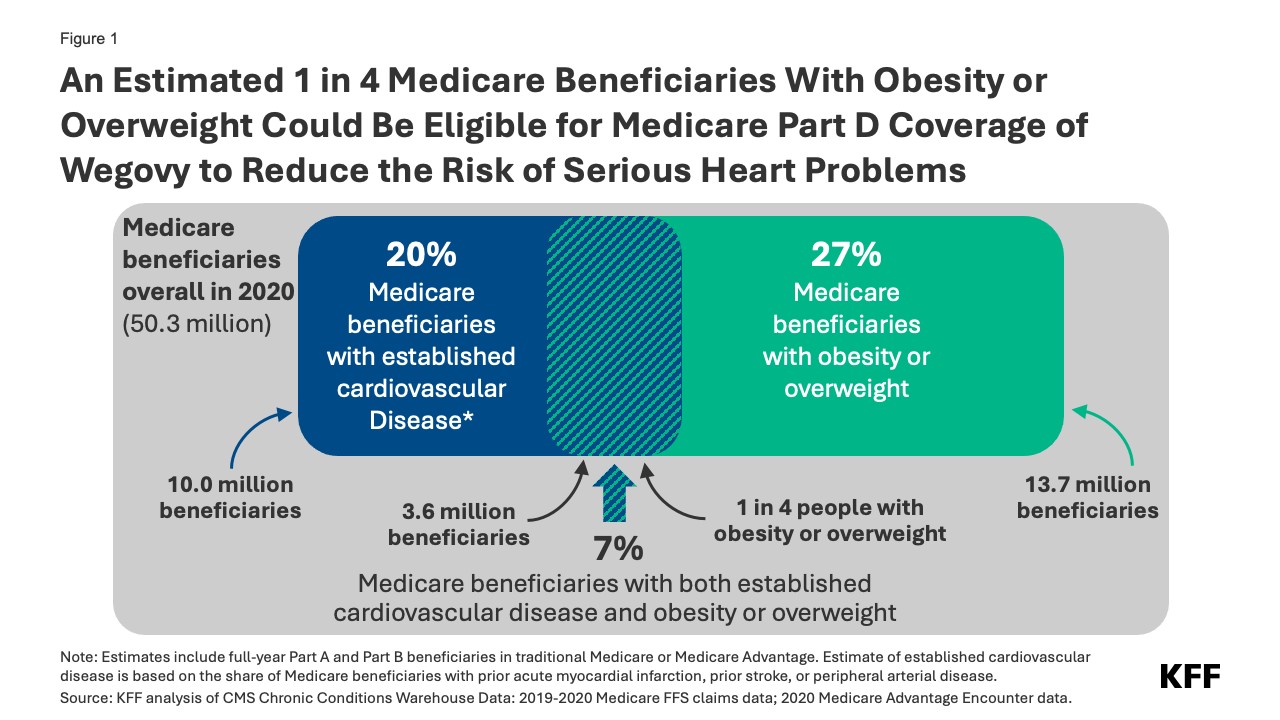 Figure 1: An Estimated 1 in 4 Medicare Beneficiaries With Obesity or Overweight Could Be Eligible for Medicare Part D Coverage of Wegovy to Reduce the Risk of Serious Heart Problems
Figure 1: An Estimated 1 in 4 Medicare Beneficiaries With Obesity or Overweight Could Be Eligible for Medicare Part D Coverage of Wegovy to Reduce the Risk of Serious Heart Problems
Notably, within this group of 3.6 million, about 1.9 million also have diabetes (excluding Type 1). These individuals might have already been eligible for Medicare coverage of GLP-1 agonists, including Ozempic, for diabetes management. The new Wegovy indication expands access based on cardiovascular risk, irrespective of diabetes status, for those meeting the cardiovascular disease and overweight/obesity criteria.
Potential Barriers: Costs and Access to Wegovy
Despite the expanded coverage potential, several factors could limit the actual uptake of Wegovy among eligible Medicare beneficiaries. Firstly, side effects and adverse reactions associated with Wegovy might deter some individuals. Secondly, cost is a significant concern. Wegovy’s list price is approximately $1,300 per month, and under Part D, it might be classified as a specialty tier drug with coinsurance rates between 25% and 33%. This could translate to monthly out-of-pocket expenses of $325 to $430 for beneficiaries before reaching annual spending caps. Even with the Inflation Reduction Act’s cap on out-of-pocket drug costs, these expenses can be substantial for many Medicare recipients, especially those with modest incomes.
Furthermore, access might be restricted by Part D plans through prior authorization and step therapy. These cost-management tools could create hurdles for beneficiaries attempting to obtain Wegovy, even if they meet the diagnostic criteria for coverage.
Timeline for Wegovy Coverage and Plan Adoption
While some Medicare Part D plans have announced intentions to cover Wegovy in the current year, widespread coverage in 2024 remains uncertain. Medicare plans can add new drugs to their formularies during the year, but they aren’t obligated to cover every new drug. Given Wegovy’s high price and the large potential patient pool, many plans might be hesitant to expand coverage rapidly, especially since they cannot adjust premiums mid-year to account for increased drug costs. Broader and more consistent coverage is more likely to emerge in 2025 as plans adjust their formularies and pricing strategies for the new coverage landscape.
Economic Impact: Medicare Spending and Wegovy
The financial implications for Medicare are substantial. The extent of increased spending will depend on several variables: how many Part D plans cover Wegovy, the stringency of utilization restrictions, the number of eligible individuals who use the drug, and the negotiated prices between plans and the drug manufacturer. If, for example, plans secure a 50% rebate on Wegovy’s list price, the net annual cost per patient could still be around $7,800. If just 10% of the estimated 360,000 eligible individuals use Wegovy for a year, the added cost to Medicare Part D could reach $2.8 billion annually for this single medication.
Looking ahead, semaglutide is a potential candidate for Medicare drug price negotiation as early as 2025. With Ozempic’s initial FDA approval dating back to 2017, it meets the criteria for negotiation. If selected, negotiated prices would take effect in 2027, potentially lowering both Medicare and patient costs for all semaglutide products, including Wegovy, Ozempic, and Rybelsus. In 2022, Medicare’s gross spending on Ozempic alone was $4.6 billion, ranking it among the top-selling drugs under Part D, highlighting the significant financial stakes involved.
Broader Implications for Anti-Obesity Medications and Medicare
For now, Medicare’s legal exclusion of weight-loss drugs remains. However, the FDA’s decision regarding Wegovy marks a significant shift. By recognizing Wegovy’s role in reducing cardiovascular risk in specific populations, it opens a pathway for Medicare coverage that circumvents the ban on obesity drugs. Further approvals for GLP-1s for other conditions could create additional avenues for Medicare coverage expansion. For instance, Eli Lilly’s Zepbound (tirzepatide) has shown promise in treating sleep apnea in overweight and obese individuals. If approved for this use, it would be the first pharmaceutical treatment for sleep apnea and could further broaden Medicare coverage possibilities for GLP-1 agonists based on diagnoses beyond diabetes and cardiovascular risk reduction.
This evolving landscape could also impact proposed legislation aimed at lifting the outright prohibition on Medicare coverage for anti-obesity drugs. As more diagnoses qualify patients for GLP-1 agonist coverage, the incremental cost of removing the statutory ban on weight-loss drugs decreases. The Congressional Budget Office would factor in the costs of these newly covered uses into baseline Medicare spending projections, making the legislative change appear less costly.
Ultimately, the expansion of Medicare Part D coverage for GLP-1 agonists, driven by new FDA-approved indications, has profound implications for individuals with obesity and related health conditions, as well as for the future trajectory of Medicare spending on these increasingly utilized medications.